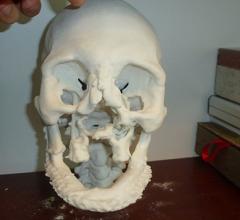
A study being presented at the Society of Interventional Radiology’s annual scientific meeting says 3-D printing could become a powerful tool in customizing interventional radiology treatments to individual patient needs.
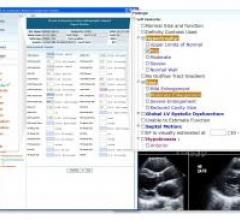
ScImage announced the immediate availability and deployment of updated quantification metrics based on guidelines published jointly in January 2015 by the American Society of Echocardiography (ASE) and the European Association of Cardiovascular Imaging (EACVI).
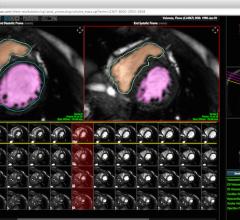
Heart Imaging Technologies announced the release of Precession, a cardiac magnetic resonance solution which allows viewing, analysis and reporting all within a standard web browser.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...

Johns Hopkins cardiologists report they have developed a formula that estimates one’s risk of dying over a decade based on a person’s ability to exercise on a treadmill at an increasing speed and incline.
New and updated American College of Radiology (ACR) Appropriateness Criteria now help healthcare providers choose the most appropriate medical imaging exam or radiation therapy for more than 1,000 clinical indications. These continually updated criteria are a national standard developed by expert panels of physicians from many different medical specialties.
CompView Medical (CVM) introduced an all-in-one equipment manager, visualization and ergonomic boom system — the NuCart.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...

Frequent use of lead aprons in the interventional lab and radiology departments to protect against radiation exposure is associated with increased musculoskeletal pain, according to a study published in the Journal of the American College of Cardiology.

Cardinal Health announced plans to acquire Johnson & Johnson’s Cordis business, a leading global manufacturer of cardiology and endovascular devices, for $1.94 billion in cash.
In a new report, SmarTech Markets Publishing forecasts shipments of 3-D printers in the medical industry will reach 2,135 units in 2020, growing to 3,055 units in 2024. The report, "3-D Printing in Medical Markets: An Opportunity Analysis and Ten-Year Forecast," shows where opportunities will be found.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
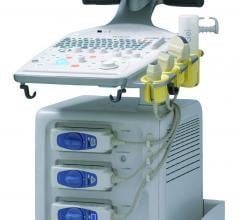
Hitachi Aloka Medical America Inc. announced the creation of a new dedicated sales force targeting cardiovascular ultrasound opportunities in physician’s offices and hospitals.
AirStrip and Life Monitor Pty Ltd. announced the formation of a strategic partnership to sell AirStrip solutions in Australia and New Zealand. Both the AirStrip ONE mobile interoperability platform and the Sense4Baby wireless fetal/maternal monitoring system will be available as part of the agreement.
The American Medical Society for Sports Medicine (AMSSM) and the FIFA Medical Assessment and Research Center (F-MARC) joined together to host the 2nd Summit on ECG Interpretation in Athletes, Feb. 26-27 in Seattle. The summit brought together top sports cardiology and sport medicine physicians from around the world to focus on improving cardiac safety in athletes and impacting sudden cardiac death.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...

Four-month-old Jackson Lane has a large scar that looks like a giant zipper down the center of his chest, the result of undergoing a lifesaving surgical procedure at just five days old to repair an extremely rare heart condition.
Merge Healthcare announced it recently acquired DR Systems Inc. for about $70 million. DR Systems is a privately held San Diego-based company in medical imaging information systems.
Nanometer-sized “drones” could become a new way to prevent heart attacks caused by atherosclerosis, according to a study in pre-clinical models by scientists at Brigham and Women’s Hospital (BWH) and Columbia University Medical Center. The drones could be used to deliver a special type of healing molecule to fat deposits in arteries, according to findings published in the February 18th online issue of Science Translational Medicine.

 March 03, 2015
March 03, 2015