For over a decade, the cardiac cryoablation industry has seen little in the way of technological advancements. Yet cryoablation, a minimally invasive procedure in which a balloon catheter delivers cryotherapy to block irregular electrical signals, is a valuable technique in treating atrial fibrillation (AF).
In the procedure, the physician deploys a cryoballoon to occlude and then ablate the pulmonary veins, freezing problematic tissue and creating scarring that blocks the irregular signals that are triggering AF. Until recently, existing technology used a single-size cryoballoon that had the potential to shift or “pop out” of the vein during an occlusion, making it difficult for physicians to ablate the targeted site. Poor occlusion could potentially result in ineffective lesions and lead to recurrence of AF that might require an additional procedure for the patient.
To make matters more complicated, pulmonary vein anatomy can vary from patient to patient, making it challenging to apply a “one size fits all” technology. Depending on the size, location and shape of the patient's vein anatomy, a larger balloon may allow for a more antral occlusion and increased tissue contact to help support effective ablation.
A Next Generation Cryoballoon
Given the well-documented limitations with conventional PVI cryoablation and based on electrophysiologist input, researchers at Boston Scientific have designed a new system for performing the procedure. The recently FDA-approved POLARxTM Cryoablation System represents significant advances in catheter, sheath and console design, all while maintaining a familiar workflow.
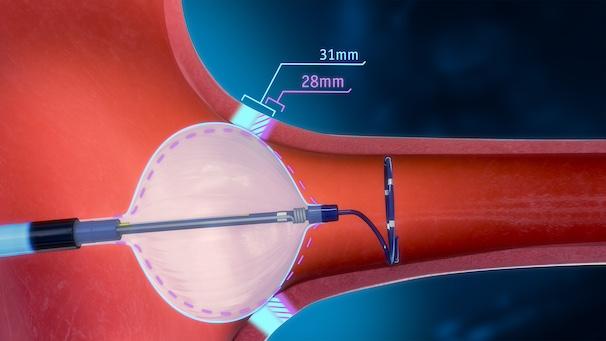
POLARx features the only available cryoablation catheter that allows a physician to switch between two balloon sizes, 28 mm and 31 mm, at the push of a button and without having to swap out catheters in the procedure. The design of the POLARx FITTM Cryoablation Balloon Catheter is based on years of physician feedback and desire for a larger cryoballoon. The POLARx FIT cryoballoon provides 20% more cooling surface area when expanded to its larger size and may offer greater occlusion capabilities, and its design may allow balloon placement in a more antral position.
The POLARx FIT cryoballoon has been demonstrated to maintain a consistent balloon size and inner pressure from inflation through ablation delivery1, a feature that can help support consistent tissue contact. This attribute is facilitated by the balloon catheter and console design. Its inner balloon pressure gauge constantly monitors pressure, allowing the console to respond and regulate it. The dual-layer balloon is made with a proprietary thermoplastic that is more compliant than the traditional technology. The combination of consistent pressure and differentiated balloon material may allow for easier placement and occlusive contact, regardless of the patient’s pulmonary vein anatomy.
Deployment and Data Collection
The POLARx system includes additional features designed to streamline cryoablation procedures. The POLARSHEATHTM Steerable Sheath has a gradually tapered tip to improve both groin and transseptal crossing. POLARSHEATH also offers an additional 20 degrees of deflection angle that could help physicians better access and align with difficult to reach anatomy, particularly the right pulmonary veins.
Once the sheath has been placed, the POLARMAPTM Circular Mapping Catheter is loaded into the POLARx FIT Balloon Catheter and delivered into the left atrium. POLARMAP is then extended from the distal end of the balloon catheter to the targeted pulmonary vein.
Once occlusion is achieved, therapy delivery can begin. As the balloon is cooled to therapeutic temperatures, the POLARMAP catheter can be used to monitor PV potentials to identify when pulmonary vein isolation is achieved. Cryoablation delivery initiates an isolation time-based dosing algorithm, if preferred and if programmed in the system, or fixed dosing can be used. The entire cryoablation procedure, from marking isolation to administering cryotherapy, can be delivered by utilizing remote control or foot pedal or by pressing the touch screen.
During the procedure, the SMARTFREEZETM Console provides real-time feedback on key procedural data points and can be controlled entirely by the physician or lab staff. This may alleviate the need for one or more staff members to manually record these metrics. The data-rich console has been designed specifically with physicians in mind, offering greater control than in previous cryoablation systems. It displays and exports clinical reports and graphs and allows for the setup of customized safety notifications to be delivered during the procedure.
Promising Results
Research studies in Europe and the United States, Canada, Australia and China have supported the safety and efficacy profile of POLARx. In the FROzEN AF global study, researchers evaluated the safety and effectiveness of the POLARx Cryoablation System for treatment of symptomatic, drug refractory, recurrent, paroxysmal AF2,3. At 12 months, data revealed a 79.9% freedom from atrial arrhythmias (88% in the FIT extension study2). The primary study cohort had a safety event rate of 4%†. Findings from a prospective, nonrandomized study conducted from 2020–2023 in experienced European centers similarly supported the safety and efficacy of the system. At 12 months, 83.5% of patients experienced no arrhythmia recurrence and 88.1% showed no recurrence of AF.4
The POLARx system has transformed a procedure that has been done in the same manner for a long time, and its design features may help improve procedural efficiencies. Recent clinical findings suggest it can help in reducing cryoablation procedure times.5
Click here for POLARx™ Cryoablation System Indications, Safety, and Warnings
Editor's note: This blog is part two of a three-part series on cardiac ablation technology. Part one, Pulsed Field Ablation: A New Ablation Method, addressed pulsed field ablation; part three will address catheter technology.
References
-
Boston Scientific bench test results. Methods: N=1 with multiple runs of each system. Test methods for inflation and ablation cycles were consistent with POLARxTM and MDT Arctic Front Cryoablation system DFUs. Bench testing performed in a 37°C saline solution bath. Bench Test results may not necessarily be indicative of clinical performance.
-
Ellenbogen, et al. One-year outcomes of pulmonary vein isolation with a novel cryoballoon in 325 patients: primary results of the FROzEN-AF trial. Presented at: Heart Rhythm Society 2023; May 19-21, 2023; New Orleans, LA, USA.
-
Su, et al. Clinical application of a novel 31 mm cryoballoon for pulmonary vein isolation for paroxysmal atrial fibrillation: procedural data from the FIT arm of FROzEN-AF. Presented at: Heart Rhythm Society 2023; May 19-21, 2023; New Orleans, LA, USA.
-
Luik A, Anic A, Asmundis C, et al. Long-term success rates of a stable, low pressure cryoballoon for the treatment of paroxysmal atrial fibrillation: Results of the prospective, international, multicenter POLAR-ICE Study. Presented at: 2023 ESC Congress, Aug. 25-28, 2023; Amsterdam, Netherlands.
-
Mojica J, Lipartiti F, Al Housari M, et al. Procedural safety and efficacy for pulmonary vein isolation with the novel POLARxTM Cryoablation System: A propensity score matched comparison with the Arctic FrontTM Cryoballoon in the setting of paroxysmal atrial fibrillation. J Atr Fibrillation. 2021 Jun 30;14(1):20200455.
† Updated analysis with corrected data.
©2023 Boston Scientific Corporation or its affiliates.
All rights reserved.
EP- 1712605-AA



 December 19, 2025
December 19, 2025 









