Five-year data suggest that the Sapien transcatheter heart valve (Edwards Lifesciences) is a feasible option for patients with severe aortic stenosis deemed to be at high risk for open-heart surgery, though valve leakage was more common with the first-generation valve evaluated in this study than with surgery, according to research from PARTNER I presented at the American College of Cardiology’s (ACC) 2015 Annual Scientific Session.

Heart failure patients with reduced ejection fraction experienced significant HF mortality and hospitalization reductions via guideline-directed medical therapy managed by pulmonary artery pressure monitoring with the CardioMEMS HF System, according to a data analysis from the CHAMPION trial.
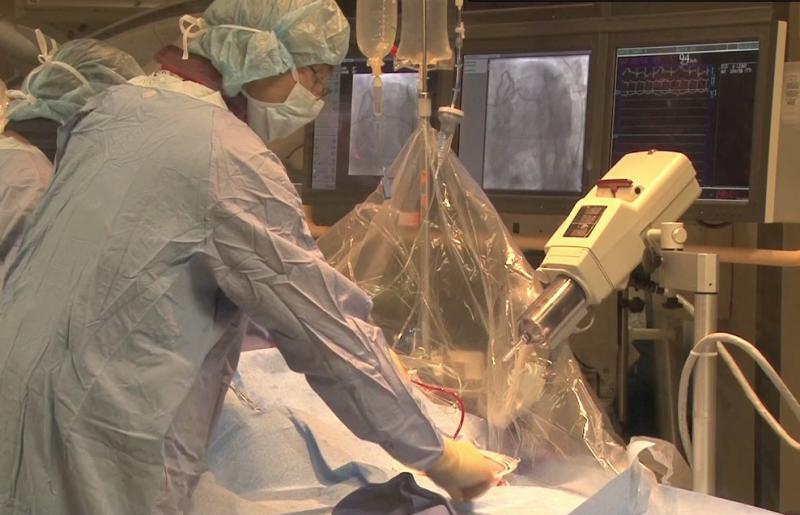
New research from the Minneapolis Heart Institute Foundation (MHIF) finds that percutaneous coronary intervention for heart attacks can still be beneficial for patients who get to their more than 12 hours after their chest pain has started. Timothy Henry, M.D., lead MHIF researcher and director of cardiology at Cedars-Sinai Medical Center in Los Angeles, presented the study results at the 2015 American College of Cardiology (ACC) conference in San Diego.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...

Results from the PROMISE clinical trial confirmed what many cardiologists and radiologists have long suspected to be true: Coronary computed tomographic angiography (coronary CTA) is extraordinarily effective in accurately diagnosing patients with low to moderate chest pain.

Medtronic plc unveiled the preclinical outcomes of its novel Drug-Filled Stent (DFS) at the 64th Annual Scientific Session of the American College of Cardiology (ACC). The preclinical data showed controlled and efficacious drug elution into the arterial wall without a polymer carrier, while reducing diameter stenosis and achieving complete stent coverage quickly without inflammation. Based on these results, Medtronic plans to initiate a clinical trial in the coming months.
Boston Scientific Corp. has received U.S. Food and Drug Administration (FDA) approval for the Watchman left atrial appendage closure device. The Watchman device offers a new stroke risk reduction option for high-risk patients with non-valvular atrial fibrillation who are seeking an alternative to long-term warfarin therapy.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Toshiba America Medical Systems Inc.’s Infinix 4DCT provides clinicians with faster, safer and more accurate interventions. Combining interventional radiology (IR) and computed tomography (CT), healthcare providers can plan, treat and verify in a single clinical setting for better patient care – rather than transferring patients between departments, risking infection and dragging out procedure times.
Precision Image Analysis (PIA) will be showcasing their remote post-processing solution for cardiac magnetic resonance (CMR) and cardiac computed tomography (CT) at the 2015 American College of Cardiology scientific session.
AtriCure Inc. announced the first two patients in the Dual Epicardial and Endocardial Procedure (DEEP) clinical study have been enrolled and treated at PinnacleHealth CardioVascular Institute in Harrisburg, Pennsylvania. The study is the first of its kind in the United States for the treatment of persistent or longstanding persistent forms of atrial fibrillation (Afib).
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Researchers at the University of North Carolina (UNC) School of Medicine have found the blood platelet protein Rasa3 is critical to the success of the common anti-platelet drug Plavix. Plavix breaks up blood clots during heart attacks and other arterial diseases.
Infraredx announced it will premiere the Advanced TVC Imaging System and the Muller NIRS-IVUS Catheter featuring Extended Bandwidth IVUS technology at the American College of Cardiology’s 2015 Annual Scientific Meeting in San Diego, March 14-16.
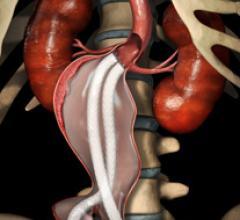
St.Vincent Heart Center is first in Indiana and second in the nation to participate in a clinical study for the Nellix EndoVascular Aneurysm Sealing System (EVAS), a surgical procedure designed to repair an abdominal aortic aneurysm (AAA).
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Women veterans who had specialized heart tests were younger and more likely to be obese, depressed and suffer from post-traumatic stress disorder than male veterans, according to a study published in an American Heart Association journal.
A new study has found that high phosphate levels can cause a stress signal inside the cells that line blood vessels, leading to the release of microparticles that promote the formation of blood clots. The findings, which appear in an upcoming issue of the Journal of the American Society of Nephrology (JASN), provide new insights into how phosphate in the diet can impact heart health.
Toshiba America Medical Systems Inc.’s Dose Tracking System makes interventional procedures safer for patients by measuring peak radiation skin dose during the exam. To expand clinical applications, Toshiba is introducing enhancements to DTS for the entire Infinix product line, including the Elite, Select and Essential cardiovascular X-ray systems.

 March 17, 2015
March 17, 2015