The U.S. Food and Drug Administration (FDA) expanded the approved use of the CoreValve self-expanding transcatheter aortic valve replacement (TAVR) system to treat certain patients who have previously had a tissue aortic valve replacement and are in need of a second one. This is the first transcatheter heart valve approved in the United States for valve-in-valve procedures in both high and extreme risk patients who have limited options or may otherwise go untreated.
The Medicines Company announced that the European Commission has granted marketing authorization for two acute care products – Kengrexal (cangrelor) and Raplixa (sealant powder). These approvals follow the issuance of positive opinions in January by the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA).
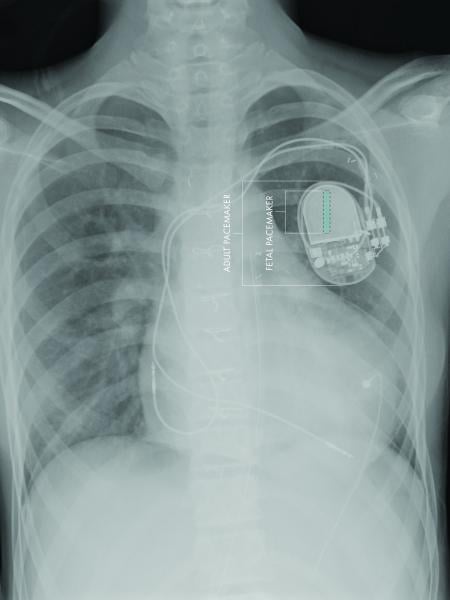
A team of investigators at Children’s Hospital Los Angeles and the University of Southern California have developed the first fully implantable micropacemaker designed for use in a fetus with complete heart block. The team has done preclinical testing and optimization as reported in a recent issue of the journal Heart Rhythm.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
AstraZeneca announced that the U.S. Food and Drug Administration (FDA) has approved a new administration option for acute coronary syndrome (ACS) patients who are unable to swallow ticagrelor (Brilinta) 90 mg tablets whole. Unlike other P2Y12 inhibitors, ticagrelor has FDA approval to be crushed and administered in water by swallowing or via nasogastric tube.
Green Circle Health (GCH) announced the availability of its GCH Platform, an online patient-to-provider communications gateway. The platform enables the real-time exchange of patient vitals and health records among physicians, patients, family members and insurers. It leverages a patent-pending workflow to facilitate the collaborative sharing of data and enhance healthcare providers’ ability to proactively monitor, diagnose and treat medical conditions.
The Aplio Platnium Series CV (cardiovascular) ultrasound systems from Toshiba America Medical Systems offer a comprehensive suite of dedicated technology for cardiac diagnosis. The series, including the Aplio 500 Platinum CV and Aplio 300 Platinum CV, feature 2-D Wall Motion Tracking, spectral and color Doppler, and Toshiba’s latest suite of guidance and workflow technologies.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Toshiba’s Aquilion One Vision Edition computed tomography (CT) system is capable of aiding clinicians in diagnosing patients presenting with chest pain. The system was showcased at the American College of Cardiology (ACC) annual meeting in San Diego, March 14-16.

Adding two non-invasive imaging tests to traditional cardiovascular disease risk factor assessment more precisely predicts a healthy patient’s future risk of heart attack, stroke, or premature death, according to a new study. The study was led by Icahn School of Medicine at Mount Sinai and published in the March 24 edition of the Journal of the American College of Cardiology (JACC).
American College of Cardiology (ACC) President Kim Allan Williams Sr., M.D., FACC, issued the following statement in response to the Senate Health, Education, Labor and Pensions Committee on “America’s Health IT Transformation: Translating the Promise of Electronic Health Records into Better Care":
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
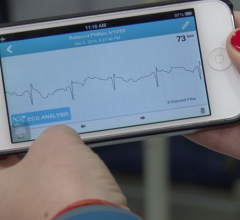
AliveCor Inc. announced the launch of a cloud-based electrocardiogram (ECG) analytics program with Preventice Solutions. Using AliveCor’s proprietary algorithms in this pilot program, Preventice Solutions intends to improve its internal workflow by reading the most critical ECGs first.
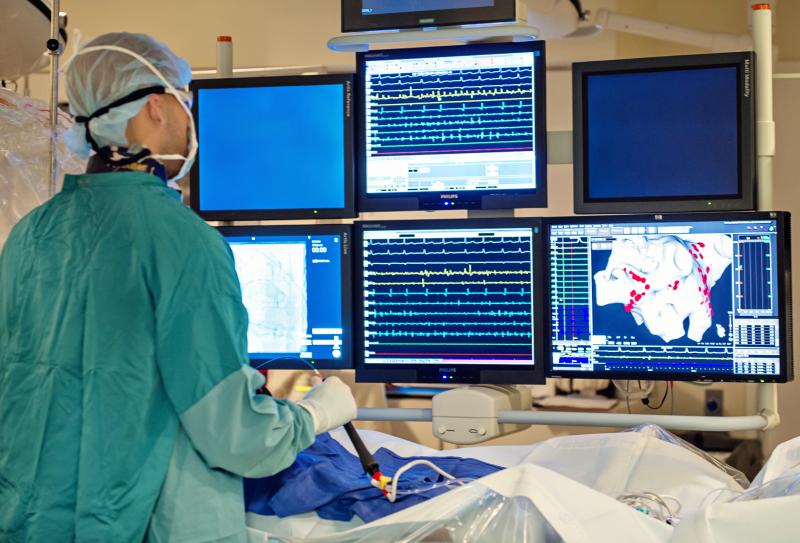
Patients with heart failure and atrial fibrillation were less likely to experience death, hospitalization or recurrent Afib with catheter ablation than with a heart rhythm-regulating drug, according to a new study. Catheter ablation was most successful in procedures where ablation was required in other areas in addition to the pulmonary vein. The study was presented at the American College of Cardiology’s 64th Annual Scientific Session.
BioTelemetry Inc. has reached a settlement agreement with the U.S. Department of Justice related to allegations that BioTelemetry encouraged physicians to use two non-specified diagnosis codes to ensure coverage of mobile cardiac telemetry. Per the agreement, BioTelemetry will pay $6.4 million to the Department of Justice, which was reflected in the Company's 2014 financial statements.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
The Society for Cardiovascular Angiography and Interventions (SCAI) 2015 Scientific Sessions will take place May 6-9 in San Diego, California. SCAI 2015 will deliver updates affecting the invasive/interventional cardiovascular field, including late-breaking clinical trials; keynote addresses; and practice-relevant education for diagnosing and treating patients with congenital, coronary, peripheral and structural heart disease.
Edwards Lifesciences Corp. announced that chairman and CEO Michael A. Mussallem served as an invited expert panelist on behalf of the Advanced Medical Technology Association (AdvaMed) at the United States Senate Health, Education, Labor and Pensions Committee hearing on "Continuing America's Leadership: Advancing Research and Development for Patients."

Research and consulting firm GlobalData reported that the global market value for transcatheter heart valves will expand at a Compound Annual Growth Rate (CAGR) of 19.7 percent through 2020, reaching an approximate value of $3.02 billion.

 March 31, 2015
March 31, 2015