The American College of Cardiology (ACC) has launched version 2.0 of their Guideline Clinical App. With the addition of the healthy lifestyle and obesity guidelines, the app now contains the full set of clinical prevention guidelines released by ACC/American Heart Association in 2013.
Merck announced results from two post-hoc analyses of the TRA 2°P TIMI 50 (Thrombin Receptor Antagonist in Secondary Prevention of Atherothrombotic Ischemic Events) trial of Zontivity (vorapaxar), one of the largest secondary prevention studies of an antiplatelet medicine.
Mountain States Health Alliance (MSHA) wanted consistency in care and data reporting from all its facilities, and through use of its CVIS it was able to greatly improve workflow, cut reporting time, reduce critical results turnaround times with chest pain patients in the emergency department, and improve quality data reporting standards.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
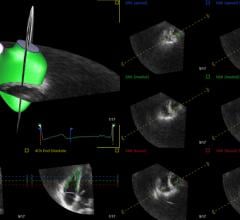
TomTec Imaging Systems GmbH will introduce its 4-D RV-Function software for right ventricular function analysis at the 64th Annual Scientific Session of the American College of Cardiology (ACC. 2015).
A three-year review of data on the Combo Dual Therapy Stent was presented at the Joint Interventional Meeting (JIM) 2015 in Rome. These data demonstrate the healing benefits of the Combo Dual Therapy Stent in the treatment of coronary artery disease in the short, medium and long-term.
Biotronik announced that its ProMRI and ProMRI AFFIRM studies have been published in Heart Rhythm, the official journal of the Heart Rhythm Society, validating the safety testing of Biotronik’s ProMRI technology.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
The Centers for Medicare and Medicaid Services (CMS) has released the 2015 National Impact Assessment of Quality Measures Report (2015 Impact Report).
A new, online report predicts where American hospitals will spend billions on healthcare technology in 2015, centering on ICD-10 migration and population health management solutions.

As an early adopter of fractional flow reserve (FFR) for the diagnosis of ischemia, my initial decision to transition ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
The continuity within clinical conference topics from year to year and how presentations augment one another tells us a lot about where medicine is headed. Pulmonary embolism (PE) therapies were a key subject presented at the 2013 and 2014 Veith Symposium meetings in New York.
The U.S. Food and Drug Administration (FDA) has approved the Xarelto Starter Pack for deep vein thrombosis (DVT) and/or pulmonary embolism (PE) treatment. The starter pack was designed to help simplify the dosing for the initial 30-day treatment period when patients are at greatest risk of having another DVT or PE.
Biotronik announced that results of its BIOLUX P-I clinical study have been published in the Journal of Endovascular Therapy. BIOLUX P-I was a prospective, international, multi-center, first-in-human, randomized, controlled trial that enrolled 60 patients at five centers in Germany and Austria. The study assessed the safety and efficacy of the Passeo-18 Lux drug-coated balloon (DCB) in treating de novo and restenotic femoropopliteal lesions. The positive results were instrumental in gaining CE mark in December 2013.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
TomTec Imaging Systems GmbH introduced AutoStrain software for automated strain analysis and quantitative visualization of left ventricular function at the 64th Annual Scientific Session of the American College of Cardiology (ACC 2015) in San Diego.
The Centers for Medicare & Medicaid Services (CMS) is working closely with stakeholders across the healthcare industry to provide support in transitioning to version 10 of the International Classification of Diseases (ICD), including an online resource. CMS will officially transition from ICD-9 to ICD-10 on Oct. 1, 2015.

Tryton Medical Inc. announced that results from the company’s pivotal clinical trial for the Tryton Side Branch Stent were published in the Journal of the American College of Cardiology. The Tryton Pivotal IDE Trial, which enrolled 704 patients at 67 centers in North America and in 11 countries in the European Union (EU) and Israel, was the largest coronary bifurcation study ever conducted.


 March 06, 2015
March 06, 2015