The spirit of collaboration to improve health with information technology came to Cleveland, Ohio, when the Integrating the Healthcare Enterprise (IHE) North American Connectathon 2015 opened in January 2015. Almost 100 organizations and 555 engineers attended this annual testing event.
Physicians who use the clinical reference tool, DynaMed from EBSCO Health, can now access the valuable, evidence-based content anywhere with the new DynaMedmobile app. The new app has been redesigned to make it easier and faster for physicians to find answers to clinical questions.

Transcatheter aortic valve replacement (TAVR) is not only a breakthough minimally invasive medical therapy, but offers a new business opportunity for hospitals that begin a program. The technology is viewed as cutting edge not only with clinicians, but also among the general public. This can be leveraged to promote the hospital as a cutting-edge facility and help attract new patients. However, creating these programs requires long-term financial, professional and equipment commitments, which may not be right for all hospitals. There are also concerns that a proliferation of TAVR centers may make programs less profitable and reduce the proficiency of any one center as patient volumes decrease if patients are spread between many competing hospitals.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Most “risk calculators” used by clinicians to gauge a patient’s chances of suffering a heart attack and guide treatment decisions appear to significantly overestimate the likelihood of a heart attack, according to results of a study by investigators at Johns Hopkins and other institutions.
Micell Technologies, Inc. announced the commercial availability of the MiStent Sirolimus Eluting Absorbable Polymer Coronary Stent System (MiStent SES) in Europe. Stentys, Micell’s distribution partner in Paris, plans a controlled launch in Western Europe followed by a full commercial launch for the second half of 2015 in selected countries within Europe, Middle East, Southeast Asia and Latin America.
Certain types of chemotherapy are associated with heart issues known as cancer therapeutics-related cardiac dysfunction. However, symptoms don’t appear until the issue is already advanced and potentially deadly. Experts at Baylor College of Medicine say early screening and monitoring using echocardiograms could be the key to helping cancer survivors stay heart-healthy and get their life back.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Thanks to a two-minute newborn screening test designed to help detect heart defects, a Michigan couple was made aware of their newborn daughter’s unexpected heart defect. The test is now a requirement in hospitals throughout Michigan.
Boston Scientific and C. R. Bard announced a deal where Boston Scientific will distribute the Lutonix 035 drug coated balloon (DCB) percutaneous transluminal angioplasty catheter in the United States under a limited distribution agreement reached with Bard. Terms of the agreement were not disclosed.
Shimadzu Medical Systems announced the first United States installation of its Trinias C-12 crossover system at First Coast Heart & Vascular Center in Jacksonville, Fla. This first North American installation of the Trinias angiographic system was completed by Shimadzu dealer/partner CMS Imaging of North Charleston, SC and began operation at the medical center in late January.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Medi-Lynx Cardiac Monitoring LLC and Medicalgorithmics have announced their participation in a research project investigating the impact of lifestyle modification on ablation outcome in atrial fibrillation (ISOLATE). Medicalgorithmics’ PocketECG cardiac monitoring system will be used for collecting and analyzing the electrocargiogram (ECG) data in the project.
Symphony Health Solutions (SHS) and the American College of Cardiology announced the completion of a new research platform. The HIPAA-compliant platform combines anonymized patient-level data from the ACC's National Cardiovascular Data Registry (NCDR) with SHS's Integrated Dataverse, which includes prescription, medical and hospital claims.
A manufacturing error caused the balloon inflation ports to be mislabeled on Covidien’s Trellis 6 and Trellis 8 Peripheral Infusion systems, prompting a U.S. Food and Drug Administration (FDA) Class I recall of the devices.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
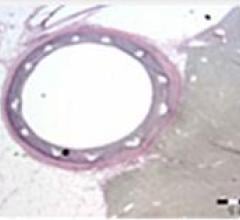
A new stent for treating cardiovascular disease that incorporates a polymer invented at Rutgers, The State University of New Jersey, has been implanted in patients for the first time.
Ablative Solutions Inc. announced the addition of Michael Weber, M.D., FACP, FACC, FAHA, to its Scientific Advisory Board.
What should doctors wear? And how does something as simple as their choice of a suit, scrubs or slacks influence how patients view them?


 February 18, 2015
February 18, 2015