Image courtesy of Reva Medical Inc.
February 12, 2015 — A new stent for treating cardiovascular disease that incorporates a polymer invented at Rutgers, The State University of New Jersey, has been implanted in patients for the first time.
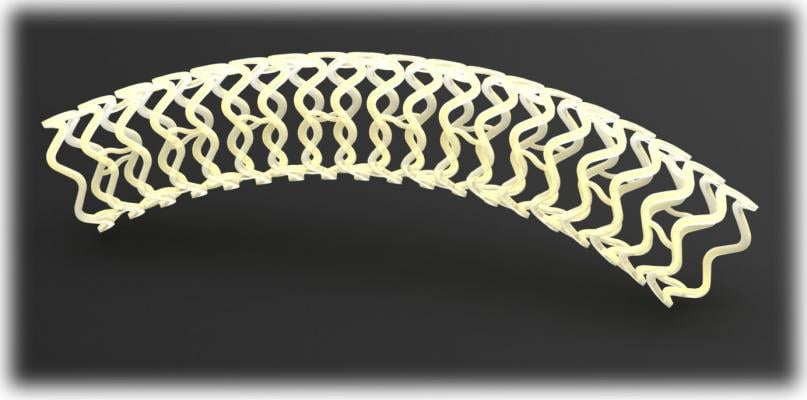
The device, called Fantom, is a drug-eluting stent being developed by Reva Medical Inc. of San Diego. The stent is made from a unique formulation from a tyrosine-based polymer family invented by Joachim Kohn, Rutgers Board of Governors professor of chemistry. The company signed the initial licensing agreement with Rutgers in 2004 for the polymer family.
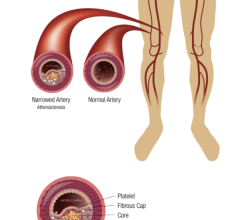
The polymer is a major step forward because the stent made from the material is fully visible using X-ray, which is extremely useful to interventional cardiologists when they are implanting the device. The polymer also is fully bioresorbable, meaning that it dissolves completely over time, which is an advantage particularly for younger patients.
The first Fantom implants were done at Institute Dante Pazzanese of Cardiology in Sao Paulo, Brazil.
“This is a major step forward toward introducing a valuable new therapeutic tool that has the potential to help many patients worldwide,” Kohn said.
For more information: www.rutgers.edu

 May 30, 2024
May 30, 2024