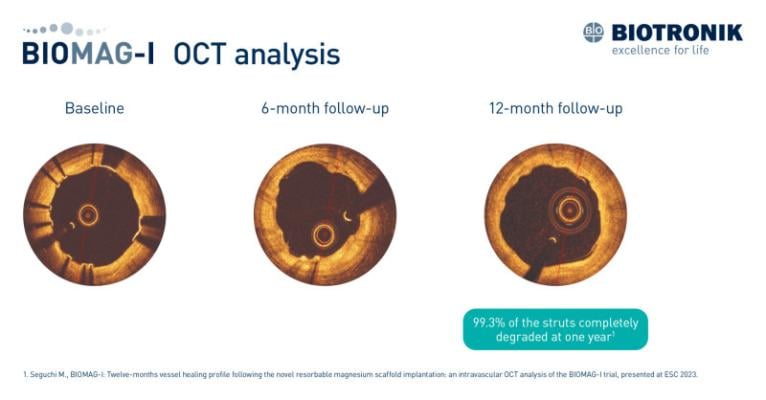
September 5, 2023 — New data from the BIOMAG-I first-in-human trial shed light on the vascular healing process following the implantation of DREAMS 3G, BIOTRONIK’s third-generation resorbable magnesium scaffold (RMS). A detailed intravascular optical coherence tomography (OCT) analysis demonstrated that 99.3% of the struts completely degraded at one year.1Dr. Masaru Seguchi from the German Heart Centre in Munich, Germany presented the findings at the European Society of Cardiology’s (ESC) congress in Amsterdam.
While earlier BIOMAG-I study results provided favorable outcomes with regards to late lumen loss at six and 12 months,2,3 the additional analysis assessed the 12-months vessel healing profile. The analysis included patients from the multicenter BIOMAG-I trial who underwent OCT imaging pre- and post-procedure, at six and 12 months. One year after implantation of DREAMS 3G RMS, the strut degradation rate was 99.3% as measured by OCT.
"We aimed to investigate the scaffold’s resorption process during the initial phase of vessel remodeling," commented Prof. Dr. Michael Joner, Deputy Director of Cardiology at the German Heart Centre in Munich, Germany. "The degradation results indicate that vascular healing following DREAMS 3G implantation appeared to be nearly complete at 12 months."
Also at the ESC congress, Prof. Dr. Michael Haude, BIOMAG-I Study Coordinating Clinical Investigator, presented another aspect of the trial. His research group investigated vasomotion after Acetylcholine (ACH) and Nitroglycerine (NTG) in 14 patients at the 12-month follow-up after device implantation. The assessment showed that vasomotion returned at one year in all tested patients.4
"The return of vasomotion indicates that the scaffolded vessel segment has been uncaged," explained Prof. Dr. Georg Nollert, Vice President Medical Affairs, Vascular Intervention at BIOTRONIK. "This supports our aim to offer a resorbable scaffold that disappears over time and leaves nothing behind but a healed vessel."
For more information: www.biotronik.com
Find more ESC23 conference coverage here
References:
1 Seguchi M., BIOMAG-I: Twelve-months vessel healing profile following the novel resorbable magnesium scaffold implantation: an intravascular OCT analysis of the BIOMAG-I trial, presented at ESC 2023.
2 Haude M., Safety and Performance of a Sirolimus Eluting Resorbable Coronary Magnesium Scaffold (DREAMS 3G): The BIOMAG-1 FIH Trial", presented at TCT 2022, ClinicalTrials.gov: NCT 04157153
3 Haude M., BIOMAG-I: One-year clinical outcome of the resorbable magnesium scaffoldDREAMS 3G, presented at EuroPCR 2023.
4 Haude M., BIOMAG-I: A new resorbable magnesium scaffold for de novo coronary lesions (DREAMS 3G): 12-month results of the BIOMAG I first in human study, presented at ESC 2023.


 November 24, 2025
November 24, 2025 









