June 4, 2024 — A patient at HonorHealth Research Institute is one of the nation’s first — and the first in Arizona and the Southwest — to undergo a non-surgical, catheter-based procedure with a new type of stroke-prevention stent that eliminates blockages in neck arteries that could potentially cause a deadly stoke by depriving the brain of oxygen.
The Neuroguard Integrated Embolic Protection (IEP) system is an experimental treatment for carotid artery stenosis, also known as carotid artery disease, a condition in which fatty-waxy deposits known as plaque builds up and blocks the normal flow of blood in the large arteries on either side of the neck. Left untreated, this condition can lead to stroke with severe complications, which could include death.
The physician who performed the procedure, Venkatesh Ramaiah, M.D., a vascular surgeon and director of HonorHealth’s vascular services, said the device avoids carotid endarterectomy, a previous method for removing plaque from carotid arteries, which involves a surgical procedure requiring an incision directly into the neck.
“This new system combines a truly innovative stent design,” said Dr. Ramaiah, a former director of the Arizona Heart Hospital. “We were fortunate to be the first in the Southwest to use such technology.”
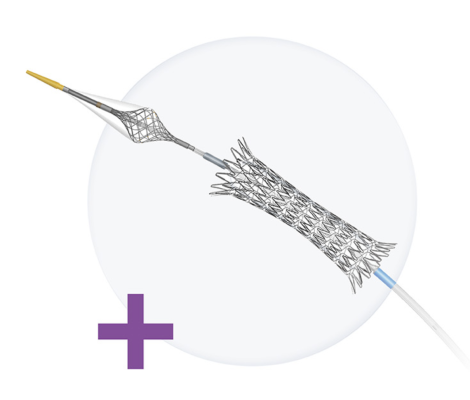
Designed by Raleigh, N.C.-based Contego Medical Inc., the Neuroguard IEP 3-in-1 carotid stent and post-dilation balloon system is designed to deliver a self-expanding carotid artery stent while using a special filter to prevent emboli (blockage) debris from reaching the brain. The stent is made of nitinol, a superelastic nickel-titanium metal alloy that is ideal for creating flexible endoscopic devices that can navigate narrow areas in the body and transform into a rigid shape when needed.
The first patient to undergo the procedure as part of the Research Institute’s Performance III clinical trial is Roslyn “Sunny” Gomes of Glendale, Ariz., who has suffered from peripheral artery disease for years. She already has three stents in her heart and so many stents and replacement arteries in both her legs that she’s lost count.
A previous scan some years ago had shown a partial blockage of her right carotid artery, but a recent set of scans showed it was 85% blocked, making her a good candidate for the new clinical trial.
“I feel a lot better today than I have,” said Sunny, 76, days after her procedure. “I really love Dr. Ramaiah. He’s been really good to me. He’s saved my right leg twice now.”
HonorHealth Research Institute is one of 30 sites worldwide to offer the device. Patients are monitored for 6 months. Enrollment in the clinical trial is expected to continue through the end of 2024.
For more information: www.honorhealth.com


 June 13, 2024
June 13, 2024 









