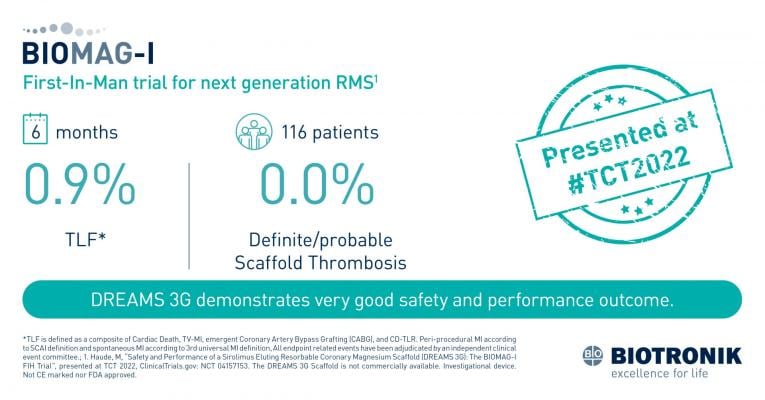
September 28, 2022 — In the "TCT Innovation" session Prof. Michael Haude, BIOMAG-I Coordinating Clinical Investigator, presented the new BIOMAG-I study results at the Transcatheter Cardiovascular Therapeutics (TCT) 2022. The multi-center study assesses the angiographic, clinical and safety performance of DREAMS 3G in patients with de novo coronary artery lesions, of BIOTRONIK’s next-generation resorbable magnesium scaffold (RMS). The data will be submitted to The Lancet.
"The excellent safety profile of previous generations was maintained with low target lesion failure rates and no scaffold thrombosis case, while the angiographic late lumen loss (LLL) is now markedly improved", said Prof. Michael Haude, Rheinland Klinikum, Neuss, Germany, adding that "the mean LLL of 0.21 mm with a median value of 0.13 mm is now compatible to the objective performance criteria defined as a median LLL of 0.18 mm for new generation DES2 in the report by the ESC task force on the evaluation of coronary stents2. This is making DREAMS 3G a potential alternative to permanent DES2."
A total of 14 clinics in eight European countries are taking part in the BIOMAG-I clinical trial. The patient cohort includes 116 patients aged between 18 and 80 years with single de novo lesions in up to two coronary arteries. The study will run for a total of five years, assessing the primary endpoint of in-scaffold late lumen loss at six and 12 months, with follow-ups on the secondary endpoints at 12, 24, 36 and 60 months.
The new scaffold is made from the BIOTRONIK’s proprietary BIOmag® magnesium alloy for improved mechanical properties3 and offers unique benefits:
- Higher radial force3
- Maintained resorption time of 12 months3
- Reduced strut thickness3
- Improved radiopacity with a new marker concept3
- Portfolio of 15 sizes to enable the treatment of a broader range of vessels and patients
"DREAMS 3G RMS combines new features that improve the physician’s practice and can address the shortcomings of DES; avoiding a permanent implant and potential late events," said Dr. Jörg Pochert, President Vascular Intervention at BIOTRONIK. "The ground-breaking BIOMAG-I data indicate that this technology could set a new standard in resorbable scaffolds."
In further news, 24-month full cohort data of the BIOSOLVE IV registry, conducted with the previous generation Magmaris RMS scaffold was presented at TCT. These results reinforced the excellent study data supporting the Magmaris RMS scaffold with low rates of target lesion failure and scaffold thrombosis.
For more information: www.biotronik.com
References:
1 Haude M., Safety and Performance of a Sirolimus Eluting Resorbable Coronary Magnesium Scaffold (DREAMS 3G): The BIOMAG-1 FIH Trial", presented at TCT 2022, ClinicalTrials.gov: NCT 04157153, submitted for publication in The Lancet.
2 Byrne R., et al European Heart Journal 2015; doi:10.1093/eurheartj/ehv203
3 BIOTRONIK data on file


 October 31, 2025
October 31, 2025 









