Agfa Healthcare announced the commercial launch of its Enterprise Imaging for Cardiology Suite and strategic relationship with TomTec to provide a unified cardiology diagnostic and clinical imaging solution. The solution will be on display at the American College of Cardiology (ACC) 2015 meeting, March 14-16, 2015 in San Diego.

Medtronic announced the results of a new "real-world" study of patients who have had a cryptogenic stroke, in which the Reveal Linq Insertable Cardiac Monitor (ICM) detected atrial fibrillation (AF) in everyday practice at an even greater rate than that found in a recent clinical trial (the CRYSTAL AF Study, which was published in The New England Journal of Medicine).
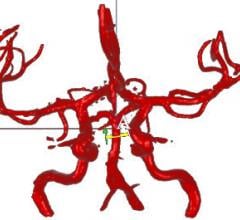
A recently completed National Institutes of Health-funded trial confirms the efficacy of blood flow measurement technology from VasSol Inc. to predict the possibility of repeated stroke in at-risk individuals.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Pediatric cardiology researchers and clinicians from numerous centers around the world gathered at the Cardiology 2015: the 18th Annual Update on Pediatric and Congenital Cardiovascular Disease conference, Feb. 11-15 in Scottsdale, Ariz. sponsored by The Children’s Hospital of Philadelphia and Phoenix Children’s Hospital.
At the 2015 annual meeting of the Healthcare Information and Management Systems Society (HIMSS), Carestream will demostrate its flexible Clinical Collaboration Platform, which now offers a new module to tag clinical data files and bring them into the workflow. These new capabilities build upon Carestream’s fully featured vendor neutral archive (VNA) architecture.
GE Healthcare initiated a voluntary field corrective action for all of its magnetic resonance imaging (MRI) systems with superconducting magnets that were manufactured from 1985 through today. The U.S. Food and Drug Administration (FDA) classified it as a Class 1 product recall Feb. 18.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Biotronik announced the completion of enrollment in the BIOSOLVE-II trial — a clinical study investigating the safety and performance of DREAMS (DRug Eluting Absorbable Metal Scaffold).
Three out of five healthcare IT professionals believe that government mandates are having a negative effect on their industry, according to a study conducted by Peak 10, a national information technology (IT) infrastructure and cloud services provider. The majority (94 percent) also noted that complying with regulations influences IT strategy and decision-making. With rare exception, respondents claimed they lack the expertise necessary to navigate the maze of government regulations.
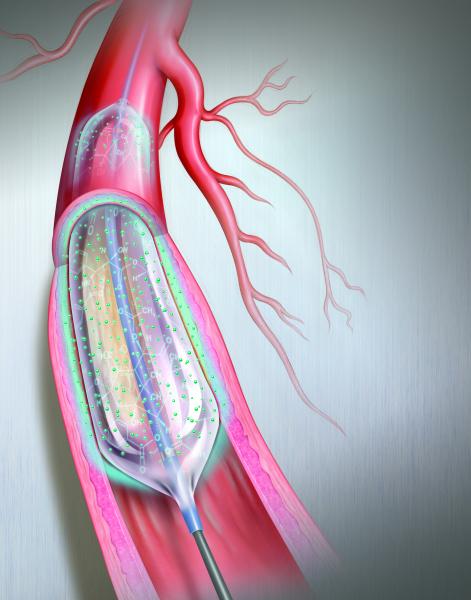
Drug-eluting balloons (DEBs) have been one of the long-awaited new transcatheter technologies to help reduce high restenosis rates in peripheral artery disease (PAD). The U.S. Food and Drug Administration (FDA) cleared C. R. Bard’s Lutonix 035 DEB in November 2014 and Medtronic’s In.Pact Admiral DEB in January 2015. Both are indicated for use in the superficial femoral and popliteal arteries in the upper leg.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
At the SPIE Medical Imaging Conference, ContextVision exhibited results from research conducted in collaboration with Texas Instruments and High Performance Consulting on 3-D adaptive filtering for improved image quality on portable medical devices. Björn Norell, research scientist at ContextVision, presented the findings.
Calgary Scientific Inc. announced the release of ResolutionMD 5.0, which offers better access to health information, supports increased teamwork and enhances communication among practitioners and patients.
Looking to “bypass the ordinary,” during last week’s episode titled: “The Bed's Too Big Without You,” the Grey’s Anatomy team at Seattle Grace Hospital was challenged with treating a radical tumor in the chest. After printing a 3-D model of the surgical area, Dr. Alex Karev commented that the best course of action to access the chest and successfully remove the mass would involve “the McGinn technique.”
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...

iRhythm Technologies Inc. announced the study, “Cost Analysis and Clinical Outcomes of Ambulatory Care Monitoring in Medicare Patients,” was published in the Journal of Health Economics and Outcomes Research. The study assessed the costs incurred in the diagnosis, additional monitoring and following clinical events after the initial use of the Holter monitor among Medicare patients with arrhythmia.
Cerner Corp. and development partner Mortara Instrument have joined together to introduce CareAware Waveform Management. The system displays waveforms and other physiological data from patient monitoring systems and integrates them into the electronic health record (EHR).

Cardiac imaging accounts for about one-third of the source of X-ray radiation dose for all medical imaging. Expanding use of computed tomography (CT) for cardiac evaluations, use of nuclear imaging for myocardial perfusion exams and more complex transcatheter procedures in the cath lab have all increased patient exposure in recent years.

 February 23, 2015
February 23, 2015