3-D Printed Heart Technology Aids Diagnosis, Procedural Planning
October 17, 2014 — Materialise NV has listed its 3-D-printed cardiovascular HeartPrint models as a medical device in U.S. and EU markets. After years of 3-D printing anatomical models for educational and research purposes, the company addressed the need for models that can assist with diagnosing, planning and practicing complex cardiovascular procedures. This move strengthens the company's position in the market and is a natural extension of its Mimics Innovation Suite of software for medical image processing, which has an existing 510(k) clearance and CE mark.
By listing HeartPrint as a Class 1 medical device, the company is able to add HeartPrint models to their offering for pre-operative planning. The 3-D-printed, patient-specific cardiovascular models are created from medical image data to provide cardiologists and surgeons with supplemental information to determine the best treatment for each patient.
"Where I think clinically 3-D printing will take us, is to the next generation of imaging. As we've seen in the history of medicine, the better and better our imaging, the more precise we are to pre-operatively be able to say what operation we're going to do," said David Morales, M.D., Chief of Cardiovascular Surgery for the Heart Institute at Cincinnati Children's Hospital Medical Center.
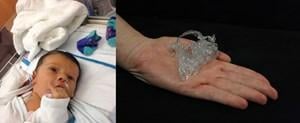
Nearly every week, the added-value of 3-D-printed solutions in the medical arena makes headlines. A recent story covered a 1-week-old baby who was born with a complex form of congenital heart disease in which both the aorta and pulmonary arteries arise from the right ventricle as well as a large hole in the heart called a ventricular septal defect (VSD). Only one day after he was born, an extremely low dose chest CT scan was acquired and data was sent to Todd Pietila, cardiovascular
business development manager at Materialise, who created a digital 3-D model of the baby's heart using Mimics and then 3-D-printed a replica where even the smallest details were visible. With the walnut-size model in hand, the team of clinicians at the New York-Presbyterian/Morgan Stanley Children's Hospital were able to find a solution for repairing all of the baby's defects in one procedure rather than the typical series of palliative operations, which can be life threatening.
"After the success of this surgery, it's hard to imagine entering an operating room for another complex case without the aid of a 3-D printed model. It's definitely going to be standard of care in the future and we're happy to be leading the way," said Emile Bacha, a congenital heart surgeon and director of congenital and pediatric cardiac surgery at NewYork-Presbyterian/Morgan Stanley Children's Hospital.
Regulatory entities have raised concerns about 3-D printing in a clinical environment as a validated quality system is critical for ensuring accuracy and safety. Materialise is the only company who is actively addressing these issues with their Mimics Innovation Suite for segmenting the medical image data and Streamics, which is dedicated to automating, controlling and tracking the 3-D printing process to ensure traceability and clinical-level quality standards.
"We're proud that the Mimics Innovation Suite is one of the few engineering packages with the appropriate validation to be considered a medical device. This makes it easier for Materialise and our customers to bring patient-specific, 3-D-printed treatments to the market. It's important for us to stay ahead of the regulatory requirements," Koen Engelborghs, director of biomedical engineering at Materialise, states. "We saw the advantages for patients when HeartPrint models were used in a clinical environment and are looking forward to continuing our collaborations with hospitals to address their 3-D printing needs."
For more information: heartprint.materialise.com

 May 12, 2020
May 12, 2020 









