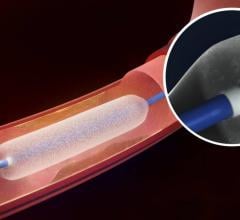
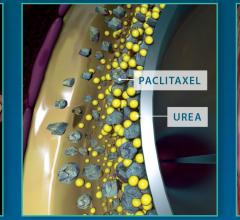
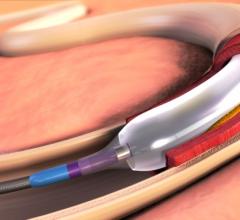
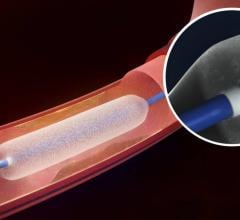
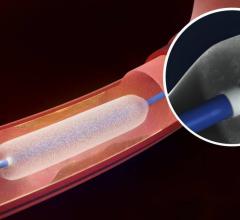
December 6, 2016 — The Spectranetics Corp. announced in late November that its Stellarex 0.014-inch drug-coated ...
Drug-Eluting Balloons
This channel includes news and new technology innovations for drug-coated balloons (DCB), also referred to as drug-eluting balloons. These are used to treat peripheral and coronary artery lesions and restenosis. The balloons carry an antiproliferative drug that is delivered to the wall of arteries when the balloon is expanded. The drug helps prevent neointimal hyperplasia (scar tissue growth) caused by trauma when the vessel segment is treated for atherosclerotic lesions with balloon angioplasty. DCBs can be used to treat hyperplasia in arteriovenous (AV) access fistulae in dialysis patients, where the vessel undergoes repeated trauma from regular punctures. DCBs also are used to treat in-stent restenosis due to scar tissue proliferation inside stents, which can cause a vessel to occlude.
September 26, 2016 — C. R. Bard Inc. announced the U.S. Food and Drug Administration (FDA) approved an Investigational ...
September 22, 2016 — New data presented at the Vascular Interventional Advances (VIVA) conference demonstrated the ...
September 19, 2016 — Medtronic plc announced that the U.S. Food and Drug Administration (FDA) approved the IN.PACT ...

Vascular Interventional Advances (VIVA) 2016 released its list late-breaking research presentations in vascular ...
July 18, 2016 — Medtronic plc has received U.S. Food and Drug Administration (FDA) approval for the IN.PACT Admiral drug ...
June 3, 2016 — Cardionovum GmbH recently announced the completion of enrollment of the RAPID trial. Results will be used ...
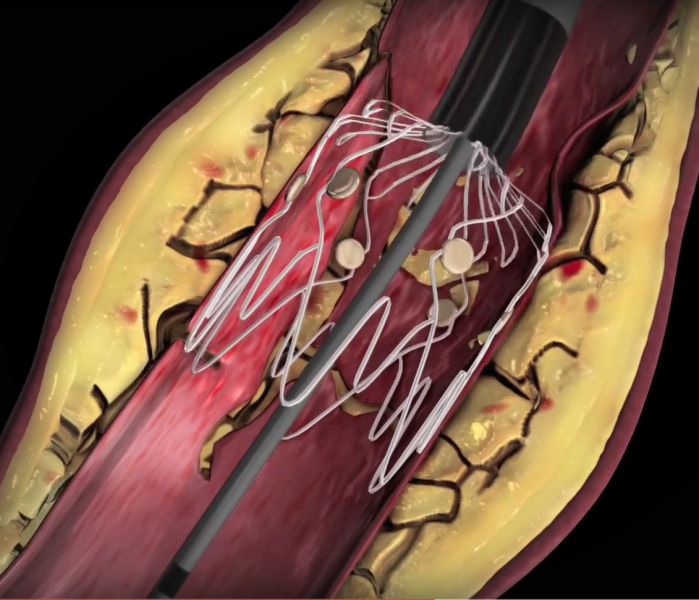
It is estimated that more than 10 million people in the United States are affected by peripheral arterial disease (PAD) ...
January 18, 2016 — Medtronic plc announced that the IN.PACT Admiral drug eluting balloon (DEB) has received CE ...
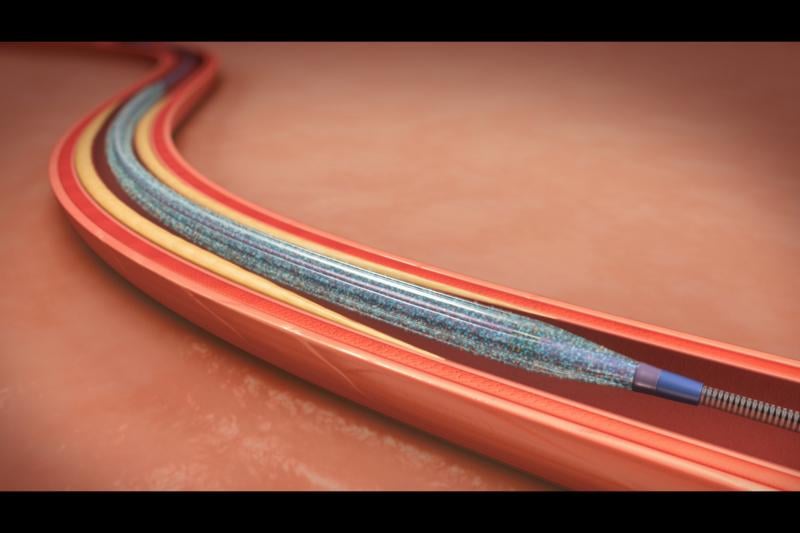
October 22, 2015 — Results from the ISAR-DESIRE 4 trial indicate that use of a scoring balloon plus a paclitaxel-coated ...

October 18, 2015 — Two-year results from the IN.PACT SFA study found the use of a drug-coated balloon was superior to co ...
October 15, 2015 — C. R. Bard Inc. announced the presentation of the 12-month results from the Lutonix Global Real-World ...
August 26, 2014 — The Cardiovascular Research Foundation (CRF) announced the late-breaking trials and first report ...
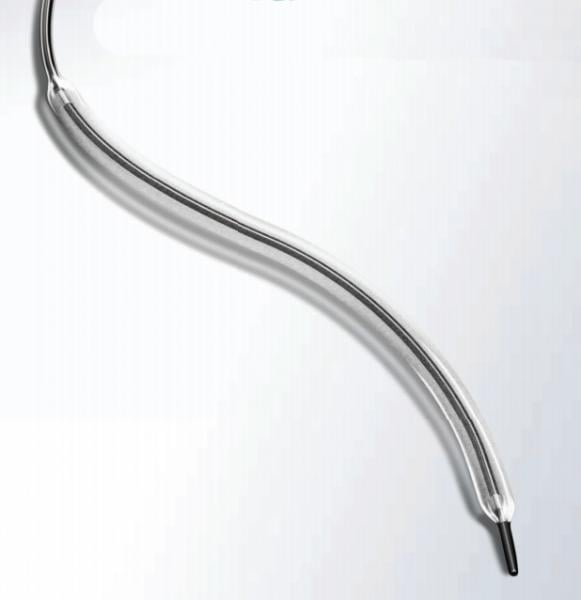
July 8, 2015 - C.R. Bard Inc. announced the publication of results from the LEVANT 2 study in the June 24, 2015, online ...
June 11, 2015 - C.R. Bard Inc. announced that the U.S. Centers for Medicare and Medicaid Services (CMS) has improved the ...


 December 06, 2016
December 06, 2016