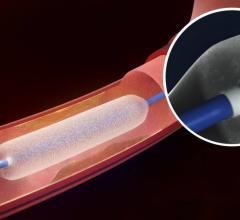
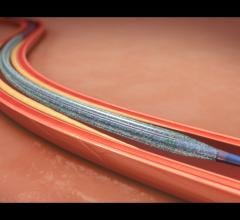
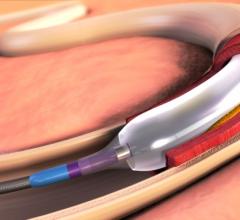
May 22, 2015 — New clinical data from two different studies show the IN.PACT Admiral drug-coated balloon from Medtronic ...
Drug-Eluting Balloons
This channel includes news and new technology innovations for drug-coated balloons (DCB), also referred to as drug-eluting balloons. These are used to treat peripheral and coronary artery lesions and restenosis. The balloons carry an antiproliferative drug that is delivered to the wall of arteries when the balloon is expanded. The drug helps prevent neointimal hyperplasia (scar tissue growth) caused by trauma when the vessel segment is treated for atherosclerotic lesions with balloon angioplasty. DCBs can be used to treat hyperplasia in arteriovenous (AV) access fistulae in dialysis patients, where the vessel undergoes repeated trauma from regular punctures. DCBs also are used to treat in-stent restenosis due to scar tissue proliferation inside stents, which can cause a vessel to occlude.
May 6, 2015 — The Spectranetics Corporation announced it is accelerating investments in the Stellarex drug-coated ...
In 2015, the FDA approved the first two drug-coated balloons for the U.S. market to treat peripheral artery disease in ...
Hear the latest trends and news on interventional cardiology from the American College of Cardiology (ACC) 2015 meeting ...
March 4, 2015 — Biotronik announced that results of its BIOLUX P-I clinical study have been published in the Journal of ...
February 23, 2015 — Medtronic announced that the U.S. Centers for Medicare and Medicaid Services (CMS) has approved a ...
February 23, 2015 — C. R. Bard Inc. announced that the U.S. Centers for Medicare and Medicaid Services (CMS) has ...
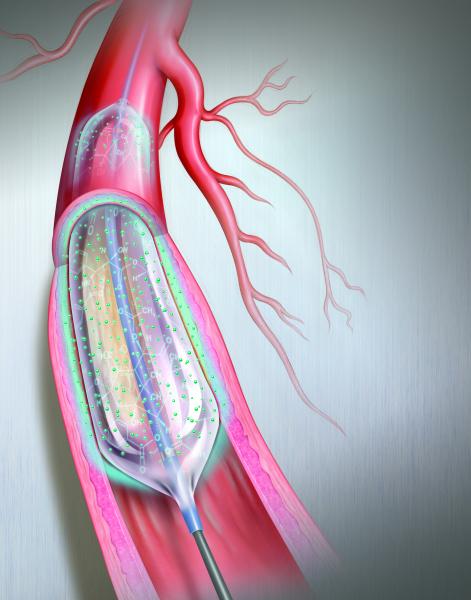
Drug-eluting balloons (DEBs), also referred to as drug-coated balloons (DCBs), have been one of the long-awaited new ...
February 13, 2015 — Boston Scientific and C. R. Bard announced a deal where Boston Scientific will distribute the ...
February 6, 2015 — United States hospitals began using a new medical device from Medtronic plc called the In.Pact ...
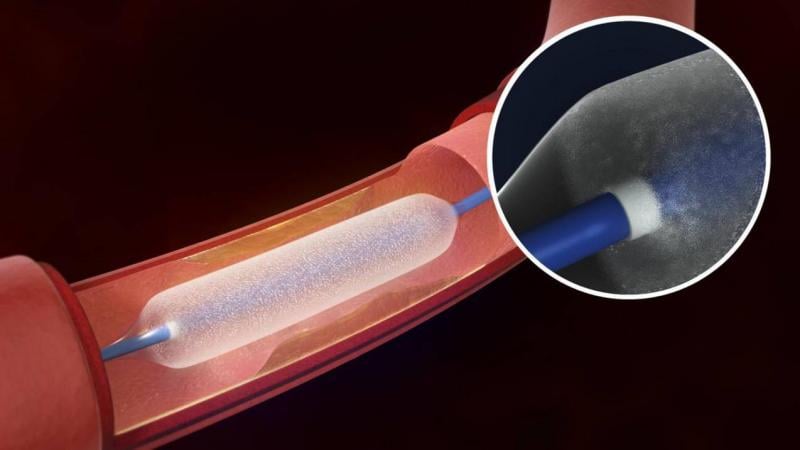
January 30, 2015 — ECRI Institute has created a report that offers an overview of drug-eluting balloon (DEB) technology ...
January 9, 2015 — Covidien announced it has received CE Mark approval for its Stellarex drug-coated angioplasty balloon ...
December 29, 2014 — The results of a study published online and in Circulation indicate that a medical device from ...
December 26, 2014 — The results of a landmark study published this month in Circulation indicate that a device from ...


 May 22, 2015
May 22, 2015