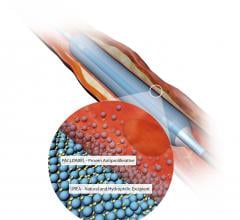
May 29, 2018 – M.A. MedAlliance SA has raised $37 million to help develop and commercialize the first sirolimus micro ...
Drug-Eluting Balloons
This channel includes news and new technology innovations for drug-coated balloons (DCB), also referred to as drug-eluting balloons. These are used to treat peripheral and coronary artery lesions and restenosis. The balloons carry an antiproliferative drug that is delivered to the wall of arteries when the balloon is expanded. The drug helps prevent neointimal hyperplasia (scar tissue growth) caused by trauma when the vessel segment is treated for atherosclerotic lesions with balloon angioplasty. DCBs can be used to treat hyperplasia in arteriovenous (AV) access fistulae in dialysis patients, where the vessel undergoes repeated trauma from regular punctures. DCBs also are used to treat in-stent restenosis due to scar tissue proliferation inside stents, which can cause a vessel to occlude.
May 3, 2018 — The U.S. Food and Drug Administration (FDA) has expanded the indication for the Medtronic In.Pact Admiral ...
February 7, 2018 — Medtronic plc recently added to its body of clinical evidence supporting the In.Pact Admiral drug ...

November 2, 2017 — The treatment of in-stent restenosis (ISR) remains challenging in clinical practice. The DARE (Drug ...
September 18, 2017 — Philips announced the two-year results from the ILLUMENATE European randomized clinical trial (EU ...
September 13, 2017 — Philips announced its presence at the Vascular Interventional Advances (VIVA 17) Annual Conference ...
September 13, 2017 — Medtronic plc announced that the In.Pact Admiral Drug-Coated Balloon (DCB) received approval from ...
August 29, 2017 — C.R. Bard Inc. announced the Lutonix 035 Drug Coated Balloon PTA Catheter (DCB) has been granted ...
August 15, 2017 — Surmodics Inc. announced receipt of an investigational device exemption (IDE) from the U.S. Food and ...
July 26, 2017 — The Spectranetics Corp. announced receipt of U.S. Food and Drug Administration (FDA) pre-market approval ...

June 28, 2017 — Philips Healthcare and Spectranetics Corp. announced they have entered into a definitive merger ...
June 12, 2017 – Med Alliance announced completion on schedule of patient enrollment in the first-in-man (FIM) study of ...
April 26, 2017 — Boston Scientific announced results from the RANGER SFA trial for the Ranger Paclitaxel-Coated PTA Ball ...
March 9, 2017 — Medtronic plc announced the launch of the IN.PACT BTK study to evaluate the effectiveness of a drug ...
February 8, 2017 — Medtronic plc announced receipt of an investigational device exemption (IDE) from the U.S. Food and ...

 May 29, 2018
May 29, 2018