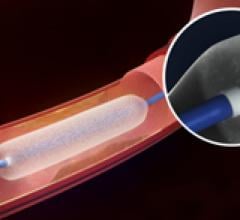
March 3, 2014 — Biotronik announced the release of its Passeo-18 Lux drug-releasing balloon (DRB) in all countries ...
Drug-Eluting Balloons
This channel includes news and new technology innovations for drug-coated balloons (DCB), also referred to as drug-eluting balloons. These are used to treat peripheral and coronary artery lesions and restenosis. The balloons carry an antiproliferative drug that is delivered to the wall of arteries when the balloon is expanded. The drug helps prevent neointimal hyperplasia (scar tissue growth) caused by trauma when the vessel segment is treated for atherosclerotic lesions with balloon angioplasty. DCBs can be used to treat hyperplasia in arteriovenous (AV) access fistulae in dialysis patients, where the vessel undergoes repeated trauma from regular punctures. DCBs also are used to treat in-stent restenosis due to scar tissue proliferation inside stents, which can cause a vessel to occlude.
Herbert Aronow, M.D., MPH, St. Joseph Mercy Hospital, Ann Arbor, and an active member of ACC and SCAI, explains the top ...

October 25, 2013 – The global sales of drug-eluting balloons (DEBs) across the 10 major markets (10MM) are expected to ...

August 19, 2013 — Medtronic Inc. announced the submission of its first pre-market approval (PMA) module to the U.S. Food ...
July 1, 2013 — One-year data from an Italian multicenter randomized controlled trial of the In.Pact Falcon drug-eluting ...
June 20, 2013 — Biosensors International Group Ltd. has entered into a licensing agreement with Eurocor GmbH, a group ...
June 10, 2013 — C. R. Bard Inc. announced the enrollment of the first patient into the Lutonix Below the Knee (BTK) Clin ...
February 11, 2013 — Vascular Nanotransfer Technologies (VNT) says it has developed the industry’s most versatile drug ...
To help identify trends and find out what DAIC readers are interested in, the magazine takes note of what they click on ...
December 26, 2012 — Covidien announced a definitive agreement to acquire CV Ingenuity. The companies expect to complete ...

 March 03, 2014
March 03, 2014