Image courtesy of C.R. Bard
October 15, 2015 — C. R. Bard Inc. announced the presentation of the 12-month results from the Lutonix Global Real-World Registry at the Transcatheter Cardiovascular Therapeutics (TCT) 2015 meeting. These results come just months after publication in the New England Journal of Medicine of the one-year data from the Lutonix 035 drug coated balloon (DCB) catheter pivotal, randomized LEVANT 2 trial.
In this registry study, the Lutonix 035 DCB demonstrated a freedom from target lesion revascularization (TLR) rate of 94.3 percent in femoropopliteal arteries at 12 months in 631 patients and, based upon interim data from 170 patients (who were early enrollees), a freedom from TLR rate of 93 percent at 24 months.
Prof. Dierk Scheinert, head of Department Interventional Angiology at the University of Leipzig in Germany, commented, “The final 12-month results of the Lutonix Global Registry demonstrated how real-world PAD [peripheral arterial disease] patients with complex and long lesions can benefit from this DCB technology without leaving metal behind. Also encouraging are the interim 24-month results which suggest durable benefits of the Lutonix DCB.”
Globally, nearly 202 million patients suffer from PAD, which if untreated could lead to serious complications or even death. According to the American Heart Association, PAD affects nearly 8 million Americans with more than 50 percent of cases involving the femoropopliteal arteries in the legs. Patients with femoropopliteal PAD have reduced blood flow to their lower extremities due to narrowed arteries and carry the risk of amputations, a cause of significant physical and psychological burden to patients and substantial costs to the healthcare system.
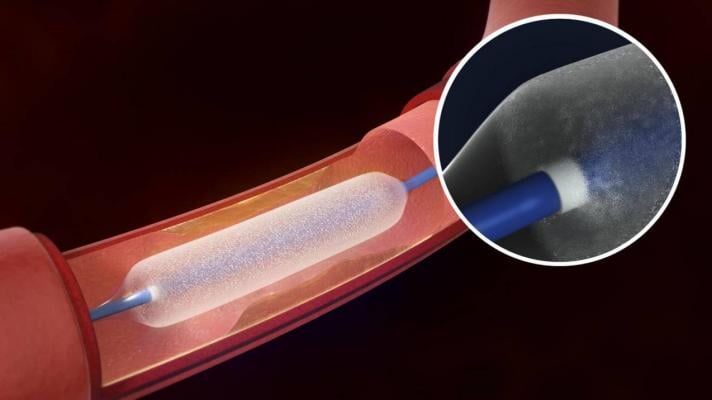
The Lutonix 035 DCB is an angioplasty balloon coated with a therapeutic dose of the drug paclitaxel, which utilizes standard mechanical dilatation to restore blood flow for patients with de novo or restenotic lesions of up to 150mm in length in native superficial femoral or popliteal arteries.
For more information: www.crbard.com


 October 31, 2025
October 31, 2025 









