On-X Life Technologies Inc. received CE marking for an expanded labeling claim of its On-X Prosthetic Heart Valve. The company may market its mechanical heart valve in Europe with areduced requirement for the use of blood-thinning drugs such as warfarin. The On-X Plus 1.5 Aortic Heart Valve allows patients to be managed at INR (international normalized ratio) levels as low as 1.5.
Aetna issued a positive coverage policy for long-term continuous monitoring of patients with suspected heart arrhythmias, which includes use of the iRhythm Technologies’ ZIO Service. The new policy now makes iRhythm’s solution – comprised of the ZIO Patch, proprietary algorithms and the ZIO report – available as a potential covered service for the insurer’s medical members.
Scientists at Icahn School of Medicine at Mount Sinai may have developed a tissue model for the human heart that can bridge the gap between animal models and human clinical trial patients. Mount Sinai researchers generated their engineered cardiac tissue from human embryonic stem cells. The resulting muscle has remarkable similarities to native heart muscle, including the ability to beat and contract like the human heart. This research study was highlighted as the cover story of the February 2014 issue of The FASEB Journal.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
CardioDx Inc. is a molecular diagnostics company specializing in cardiovascular genomics. It published an analysis demonstrating that the Corus CAD gene expression score is significantly associated with plaque burden and luminal stenosis. The holds true in symptomatic patients being evaluated for obstructive coronary artery disease (CAD), as measured by CT-angiography. The study, "A Peripheral Blood Gene Expression Score is Associated with Atherosclerotic Plaque Burden and Stenosis by Cardiovascular CT-Angiography," appears online in the journal Atherosclerosis.
Biotronik, a manufacturer of implantable cardiac devices and wireless remote monitoring technologies, has been added to the list of cardiac rhythm management (CRM) device suppliers to the U.S. Department of Veterans Affairs (VA).

Advancements in technologies are paving the way for many healthcare providers to connect with patients outside their hospitals more quickly and efficiently. For cardiology departments, technologies that allow for video collaboration, rapid transmission of scans and images and other forms of telemedicine are supporting the initiative to improve productivity, an initiative many are striving for as the U.S. healthcare landscape continues reform.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
At Johnston Medical Center in Smithfield N.C., StatVideo’s EchoCart streams real-time images of babies’ hearts and live video conferencing over the web to pediatric cardiologists at Duke Children’s Hospital. EchoCart is a telemedicine system designed specifically for tele-echocardiography.
Vascular Solutions Inc. launched the ThrombiDisc topical hemostat designed for use around indwelling lines up to 12°F. ThrombiDisc contains both the power of thrombin to facilitate hemostasis and the antimicrobial properties of silver.
After several months of intense review and assessment of its specialized worker training program by an independent agency, Operon Resource Management of Lowell, Mass. received ISO 13485 certification.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
A University of Utah-led study for treatment of patients with atrial fibrillation (A-fib) provides strong clinical evidence for the use of 3-D MRI to individualize disease management and improve outcomes. Results of the Delayed-Enhancement MRI Determinant of Successful Radio-frequency Catheter Ablation of Atrial Fibrillation (DECAFF) study will be published in the Journal of the American Medical Association.
eCardio Diagnostics is opening its second Independent Diagnostic Testing Facility (IDTF) in San Francisco, Calif. Its first IDTF is located in Houston, Texas.
Results from several late breaking clinical trials will be presented during the American College of Cardiology (ACC) 2014 annual meeting March 29-31. These will be featured during five late-breaking clinical trial sessions.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
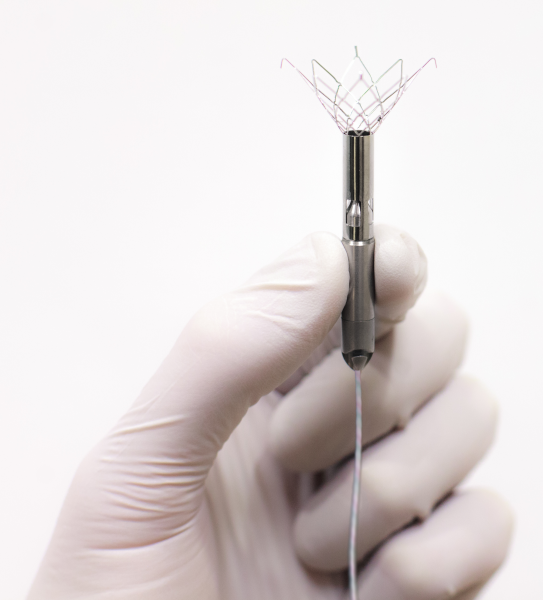
Start-up company Procyrion Inc. is developing a catheter-deployed circulatory assist device intended for long-term use in the treatment of chronic heart failure. The 6 mm diameter Aortix device is narrower than a pencil and is delivered via a catheter in a minimally invasive outpatient procedure lasting about 10 minutes.
The American College of Radiology (ACR) strongly supports the bicameral, bipartisan legislation to replace the sustainable growth rate (SGR) payment formula. The organization particularly applauds inclusion of several ACR backed provisions that raise quality of care, make care more efficient and increase transparency in physician payment policy.

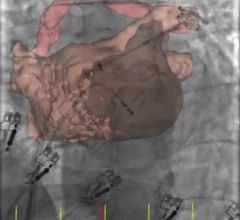
Doctors at Henry Ford Hospital in Detroit used an Edwards Lifesciences Sapien transcatheter aortic valve replacement (TAVR) device to repair a mitral valve that was narrowed with calcium buildup. William O'Neill, M.D., medical director of the Center for Structural Heart Disease at Henry Ford Hospital, estimates this new technique could help thousands of patients a year in the United States.


 February 10, 2014
February 10, 2014