Royal Philips’s IntelliSpace Portal was named a Category Leader in Enterprise Advanced Visualization Software in the 2013 Best in KLAS: Software and Services report.
March 3, 2014 — OrbusNeich announced that patient enrollment has initiated in Japan in the pivotal clinical trial of the Combo dual-therapy stent, employing a single Japan-U.S. protocol conducted as a global clinical trial “proof-of-concept” under the framework of the joint Japan-U.S. Harmonization-By-Doing [HBD] initiative.
Evena Medical, a developer of high-definition imaging for precision venous access, announced an agreement with a major distribution partner to distribute its products in 15 countries in the Middle East and North Africa. Evena also received a $5 million tranche of the company’s planned $15 million Series B financing. Evena featured its Eyes-On Glasses at HIMSS14 in Orlando, Fla.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
The Medtronic SureScan pacing systems are now approved for MRI scans positioned on any region of the body. The U.S. Food and Drug Administration (FDA) previously approved the SureScan for use with magnetic resonance imaging (MRI). Patients implanted with the Advisa DR MRI or Revo MRI SureScan pacing systems now can have MRI scans without positioning restrictions, including the chest area, which was previously restricted.
Honeywell expanded its Captuvo line of encasements for Apple devices at the HIMSS 2014 Annual Conference & Exposition in Orlando, Fla. The Captuvo SL22h and SL42h are designed for healthcare environments and equip iPod touch (5th generation) and iPhone (5, 5c and 5s) devices. The barcode data collection can increase productivity and support healthcare “Converged Device” strategies.
Accusoft released the Barcode Xpress Mobile software development kits (SDKs) for iOS and Android devices. The SDK empowers developers to integrate mobile device barcode recognition into existing apps, or to build apps based on the sample code included with the kit.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
AirStrip announced the launch of AirStrip One Cardiology for all hardware using the Windows 8.1 operating system. This includes the Microsoft Surface tablet and a variety of other mobile devices, as well as laptops and desktops.
The U.S. Food and Drug Administration (FDA) cleared Admedus’s CardioCel, a tissue product to repair and treat a range of cardiovascular and vascular defects. The company is looking to complement its existing product launch in Europe with preparation for initial sales in the United States.
The University of Michigan’s Center for Integrative Research in Critical Care (MCIRCC) has entered into a letter of intent with AirStrip with the goal of executing future collaborations, and MedStar Health has entered into a collaboration agreement with AirStrip. Both agreements are part of the AirStrip Innovation Marketplace (AIM) Program.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Scripps Green Hospital has become the first hospital in the United States to implant the world's smallest implantable cardiac monitoring device. Scripps Clinic cardiologist John Rogers, M.D., successfully completed the first implant of the Reveal Linq Insertable Cardiac Monitor (ICM) in 71-year-old San Diego resident Chuck Beal.
AliveCor Inc. announced a partnership with Practice Fusion, a physician-patient community. Physician offices will now be able to import the AliveCor Heart Monitor’s ECG readings, annotated reports and reviews into the Practice Fusion HER. It an attempt to allow them to see a more complete picture of their patients’ medical history and make more educated treatment decisions. Practice Fusion's EHR is used by more than 100,000 medical professionals on a monthly basis, and all of whom will now be offered the ability to import AliveCor ECGs directly into their EHR. Through the AliveECG app physicians can record a patient’s ECG, obtain an expert review, annotate and electronically transfer this data into the EHR within seconds.
Cordis Corp. announced an agreement with TriReme Medical Inc. that grants the company exclusive distribution rights for the Chocolate PTA Balloon Catheter.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
The U.S. Food and Drug Administration (FDA) approved Biosense Webster Inc.’s Thermocool Smarttouch Catheter. The therapeutic catheter enables direct and real-time measurement of contact force during catheter ablation procedures for patients suffering from drug-resistant paroxysmal atrial fibrillation (Afib), sustained monomorphic ischemic ventricular tachycardia and Type I atrial flutter.
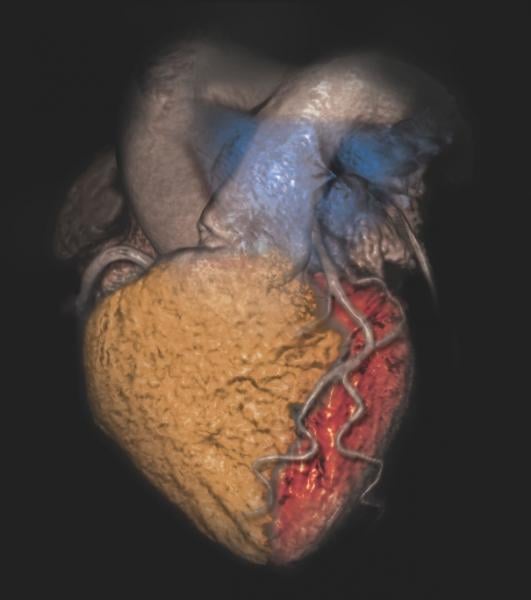
Determining the appropriate use of cardiovascular imaging requires analyzing the “complex interplay” among care processes and their quality, patient health outcomes and medical costs, according to a health policy statement released by the American College of Cardiology and endorsed by 14 other relevant medical societies.
February 28, 2014 — Lexmark’s Perceptive Software demonstrated its Universal Clinical Platform solution for enterprise clinical content management at the 2014 meeting of the Healthcare Information and Management Systems Society (HIMSS).


 March 03, 2014
March 03, 2014