An independent study from the University of Pittsburgh Medical Center, published online this week in EP EuroPace, showed significant differences in battery longevity between contemporary cardiac resynchronization therapy defibrillator (CRT-D) devices, and that the Boston Scientific Corp. device has the longest battery life compared to similar brands.

Since its debut in 2010, the renal denervation market has generated enthusiasm across the medical device industry with predictions that the market would reach more than $1 billion by 2020. However, the impact of major barriers, which will slow down this growth, was essentially underestimated according to an analyst with research and consulting firm GlobalData.
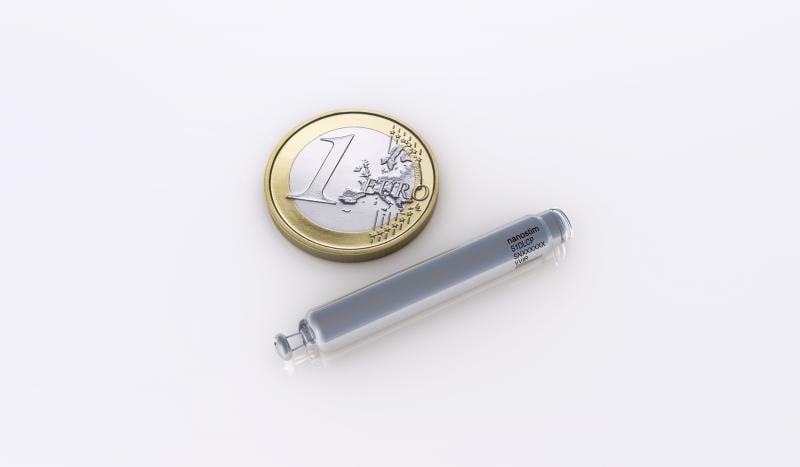
St. Jude Medical Inc. announced the completion of its acquisition of Nanostim Inc., developer of a miniaturized, leadless pacemaker, and that the device received CE mark clearance in Europe.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Digisonics Inc. introduced functionality for appropriate use criteria (AUC) calculations. Digisonics recognizes that reimbursement audits will be tied to appropriate use scores in the future, benefitting facilities that utilize the Digisonics CVIS (cardiovascular imaging and information systems) to monitor appropriate use scores and produce the required structured reports.
The Society for Cardiovascular Angiography and Interventions (SCAI) assembled a panel that has proposed new criteria for identifying patients who experience a heart attack after coronary angioplasty or bypass surgery. The new criteria and their implications for patient care are detailed in an expert consensus document e-published today in Catheterization and Cardiovascular Interventions (CCI) and the Journal of the American College of Cardiology (JACC).
The U.S. Food and Drug Administration (FDA) has cleared Sotera Wireless Inc.’s patented continuous non-invasive blood pressure (cNIBP) technology, a new feature of the ViSi Mobile wireless patient monitoring system. For the first time clinicians can continuously monitor all patient vital signs, including beat-to-beat blood pressure, without the use of a catheter or blood pressure cuff.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
The Boston Scientific OffRoad Re-Entry Catheter System demonstrated excellent performance in facilitating the treatment of complete blockages in the major arteries that supply blood to the legs. These blockages, called chronic total occlusions (CTOs), are often associated with peripheral artery disease (PAD). The data from the Re-ROUTE clinical trial were reported in a late-breaking clinical trial session at the Vascular Interventional Advances Conference (VIVA) in Las Vegas.
AccessClosure Inc. announced an exclusive agreement with Ostial Corp. to distribute the Flash Ostial System Dual Balloon Angioplasty Catheter in the United States. The system is designed to help overcome the challenges of aorto-ostial stenting and complements the Mynx product family of vascular closure devices.
CardioDx Inc. announced results of two studies indicating that Corus CAD, a blood-based gene expression test, may help reduce unnecessary cardiac testing and costs by aiding clinician decision-making in the evaluation of women with obstructive coronary artery disease (CAD) symptoms. The studies were presented at The North American Menopause Society (NAMS) 2013 Annual Meeting in Dallas, Texas.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...

High levels of high-density lipoprotein (HDL) have been linked to increased breast cancer risks and enhanced cancer aggressiveness in animal experiments.
Preventice Inc. has received CE mark certification for its BodyGuardian remote monitoring system.
Bioventrix announced the presentation of scientific data demonstrating the durability of its Revivent Myocardial Anchoring System in 24 patients one year post-procedure. The data, which Andrew Wechsler, M.D. and professor of cardiothoracic surgery, Drexel University, presented at the prestigious European Association for Cardio-Thoracic Surgery meeting in Vienna, Austria, highlighted results from the company’s Phase I clinical trial conducted at five European centers.
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...

A mother and her 25-week-old fetus are doing well after a team of physicians performed a successful in utero cardiac interventional procedure on the fetus at California Hospital Assn. (CHA) Hollywood Presbyterian Medical Center.
Mortara Instrument Inc. announced an integrated connectivity solution that provides data from the Quinton Q-Tel Rehabilitation Management Systems to the American Association of Cardiovascular and Pulmonary Rehabilitation’s (AACVPR’s) Outpatient Cardiac Rehabilitation Registry. The web-based registry is the first of its kind in the United States and will provide cardiac rehabilitation professionals with a means to track, document and communicate patient outcomes and program performance.
Critical Diagnostics announced that results of the Heart Failure: A Controlled Trial Investigating Outcomes of Exercise (HF-Action) study published in the American Heart Association’s (AHA’s) Circulation: Heart Failure demonstrates that levels of the biomarker ST2 are predictive of long-term outcomes for people suffering with heart failure and identify those patients who may benefit from exercise.


 October 14, 2013
October 14, 2013












