Transcatheter Technologies GmbH, a medical device company, is developing a third-generation transcatheter aortic valve implantation (TAVI) system, Trinity. An independent laboratory completed advanced wear testing (AWT) of the company’s Trinity valve prosthesis. AWT of the Trinity heart valve has completed 600 million cycles, or an estimated 15 years of durability testing.

The topic of radiation safety and radiation dose monitoring has moved from state-specific regulations to a national trend with The Joint Commission’s (TJCs) recent announcement of their “New and Revised Diagnostic Imaging Standards.” The call for dose management and tracking has graduated from being advised to being mandated – from both a legal perspective and from within the world of healthcare’s patient safety foundation. The question that many organizations find themselves asking is “Where does this leave me?” and “Are we prepared for compliance?”
To address the needs of physicians who treat patients with valvular heart disease, Esaote North America established a sales force to bring 3mensio Structural Heart software for cardiovascular planning solutions.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Boston Scientific launched in the United States the OffRoad Re-Entry Catheter System to treat complete arterial blockages in the major arteries of the legs. Chronic total occlusions (CTOs), are associated with advanced peripheral artery disease (PAD).
New electrophysiology (EP) ablation mapping/navigation systems recently entered the U.S. market, each offering technology the vendors say will speed procedure time and improve procedural accuracy.
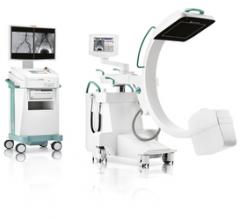
Ziehm Imaging received U.S. Food and Drug Administration (FDA) clearance to market its new generation Ziehm Vision RFD C-arm for pediatric and other interventional operating room procedures. The Ziehm Vision RFD C-arm offers fully motorized movement on four axes to maximize image quality while minimizing procedural dose. It was cleared for a range of image procedures including specific intended uses in pediatric imaging.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
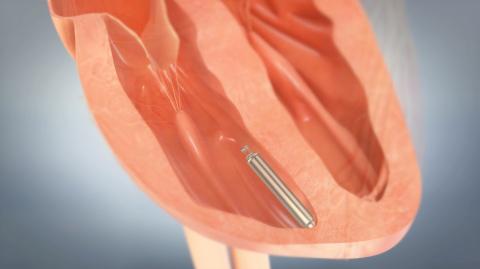
The first U.S. implant was announced for St. Jude Medical’s LEADLESS II pivotal trial, designed to evaluate the Nanostim leadless pacemaker for U.S. Food and Drug Administration (FDA) approval. The world’s first retrievable, non-surgical pacemaker was implanted at The Mount Sinai Hospital in New York City by Vivek Reddy, M.D.
The average age of installed MRI scanners in the United States has increased from 8.7 years in 2010 to 11.4 years in 2013, according to a new market research report by IMV Medical Information Division.
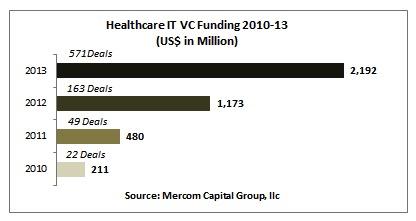
Mercom Capital Group LLC, a global communications and consulting firm, released its annual report on funding and mergers and acquisition (M&A) activity for the healthcare information technology (IT) sector in 2013.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...

GlobalMed’s CapSure Cloud application can eliminate a second radiation dose by making an initial CT scan available to all healthcare providers involved in a patient’s care. Once the images are uploaded to the secure cloud imaging server from the originating hospital, the sign-on information can be shared with specialists at the receiving hospital so they can view the study. In emergency cases, this means the team at the receiving hospital can be prepared for the patient’s arrival and go directly to the OR.
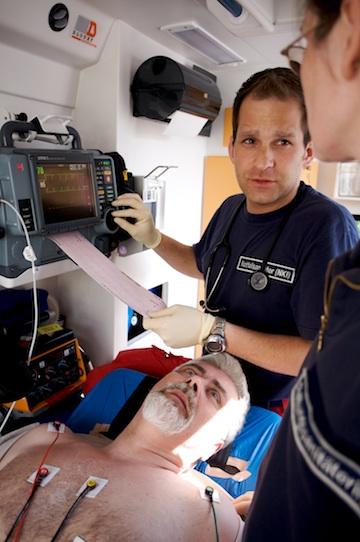
For U.S. hospitals and emergency medical services (EMS) looking to upgrade their aging defibrillator-monitors, new technologies added to these systems enable more feedback to first responders and can help speed door-to-balloon times by transmitting pre-hospital ECGs.
Percutaneous coronary intervention (PCI) centers are unequal relative to population and heart attack prevalence across the United States, according to a study in the Journal of the American Heart Association. A multi-center team led by James Langabeer II, Ph.D., The University of Texas Health Science Center at Houston, conducted the study.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
InfoBionic’s MoMe System for cardiac arrhythmia monitoring received CE marking. The MoMe System is a remote patient monitoring platform that can transition between Holter, Event and Mobile Cardiac Telemetry (MCT) modes. The platform leverages cloud computing and proprietary analytics, and delivers on-demand access to patient data for diagnosis.

Use of therapeutic hypothermia is used to prevent neurological damage in patients who suffer sudden cardiac arrest (SCA), and there are many advocates suggesting a similar approach can help improve outcomes in ST-elevated myocardial infarction (STEMI) patients.
ImaCor Inc., developer of hemodynamic transesophageal echocardiography (hTEE) technology, released the hTEE QuickStartT mobile application. The app complements ImaCor’s hTEE training program and provides clinicians access to how-to videos, patient case reviews and customer support.


 February 06, 2014
February 06, 2014