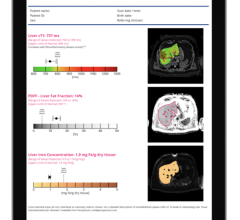
October 3, 2013 — When echocardiograms prove insufficient for diagnosis, Diagnosoft’s offers a patented and U.S. Food and Drug Administration (FDA) 510(k)-cleared cardiac magnetic resonance imaging (CMR) solution for patient assessment. With benchmark sensitivity and specificity, the high throughput Diagnosoft CMR exam yields an advantageous alternative to nuclear or cardiac cath exams.
Its automated analysis, Progressive Adoption Program and echo-centric report production make Diagnosoft CMR practical for both hospital-based and freestanding facilities.
The software is designed to help facilities improve patient experience and outcomes and contain cardiac imaging costs. It also delivers the unequivocal cardiac assessment to patients and referrals.
For more information: www.diagnosoft.com


 January 21, 2026
January 21, 2026