Orlando Heart Specialists (OHS) has added an 8th location in southwest Orlando, Fla.
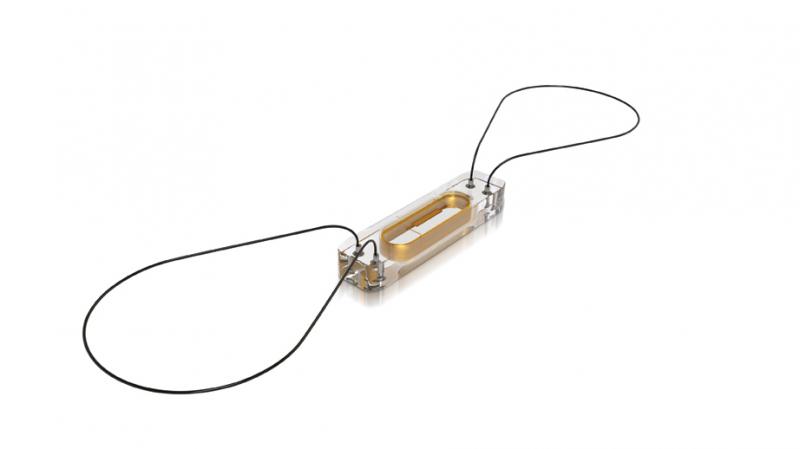
Biotronik announced that the first device patients to undergo full-body magnetic resonance imaging (MRI) scans in the United States have been implanted with the Biotronik DX System.

This August marks the 100th anniversary of the start of World War I in 1914. A few months after the start of the war, in early 1915, the Germans began using Zeppelins in the first application of strategic bombing over British cities. Zeppelin raids continued over Russia, Belgium, France, Italy and Britain for the duration of the war. These raids ironically paved the way for U.S. government policy that guaranteed access to the cryogenic gas required to operate today’s magnetic resonance imaging (MRI) systems.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
In the current era of healthcare reform with the overarching goal of reducing healthcare costs, heart failure (HF) management has taken center stage. It is the leading cause of hospitalization for adults over the age of 65 in the United States and accounts for more than 1 million hospitalizations annually, with Medicare expenditures of more than $17 billion.[1,2] It is apparent that current HF monitoring efforts are not adequate, which has led to great interest in more effective technologies.
A big fly in the ointment for widespread adoption of many new technologies is cost. In today’s cost-conscience environment of healthcare reform, there needs to be a clear, quantifiable return of investment (ROI).
Mr. KH is a 58-year-old male with a past medical history of hypertension and tobacco abuse who presented to the emergency department (ED) with an episode of syncope. The patient stated that he was at home walking to the kitchen when he felt light-headed and then woke up on the floor. The event was witnessed by his wife, and he was not noted to have any seizure-like activity. On arrival in the ED, the patient began to complain of chest discomfort and dyspnea. A stat electrocardiogram (ECG) revealed sinus rhythm and 2 mm ST-segment depression in leads V4-V6. The patient lost consciousness while in the ED and was emergently intubated. His blood pressure was 65/45, and he was started on intravenous phenylephrine, dopamine and norepinephrine to maintain a blood pressure of 70/40. The patient was taken emergently to the cardiac catheterization laboratory (CCL) where an intra-aortic balloon pump (IABP), was emergently placed. Coronary angiography revealed mild atherosclerotic disease and the aortic valve could not be crossed.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
July 8, 2014 — NorthStar Medical Radioisotopes LLC has signed a pledge with the Preparatory Commission for the Comprehensive Nuclear-Test-Ban Treaty Organization (CTBTO), designed to help the CTBTO detect nuclear testing.
July 8, 2014 — A Huntington Memorial Hospital patient has become the first patient in the San Gabriel Valley in southern California to be implanted with a tiny, leadless cardiac pacemaker. Developed for patients with bradycardia, St. Jude Medical’s Nanostim device is designed to be placed directly in a patient’s heart without the visible lump, scar and insulated wires (leads) required for conventional pacemakers.
July 8, 2014 — Humana Inc. announced earlier this year an expanded coverage policy for cardiac CT (computed tomography). Many of the new Humana indications have long been advocated for by the Society of Cardiovascular Computed Tomography (SCCT) and described in the model local coverage determination policy (LCD) developed by the organization.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Use of catheter ablation is not only beneficial for treating atrial flutter but also can significantly reduce hospital visits — both inpatient and emergency — and lower the risk for atrial fibrillation, according to research by the University of California San Francisco (UCSF).
CardiAQ Valve Technologies (CardiAQ), a leader in the field of transcatheter mitral valve implantation (TMVI), announced that the European Patent Office Legal Division issued a temporary stay of further proceedings in Neovasc’s pending European patent application covering TMVI technology.
Thoratec Corp. has acquired Apica Cardiovascular Ltd. for an upfront cash payment of $35 million and potential future clinical and sales milestones of up to $40 million.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Vital Connect announced its HealthPatch MD biosensor has received U.S. Food and Drug Administration (FDA) clearance. The solution is capable of capturing clinical-grade biometric measurements and is the first FDA-cleared medical device that includes a fall detection feature.
Corindus Vascular Robotics has been named the recipient of the 2014 North American New Product Innovation Award in Interventional Cardiology, which was established by Frost & Sullivan to recognize visionary innovation and product excellence.
Vital Images Inc. announced the global release of VitreaExtend, an advanced visualization solution that supports up to three simultaneous users with high performance similar to Vital's advanced server-based solution.

 July 10, 2014
July 10, 2014











