Accusoft released the Barcode Xpress Mobile software development kits (SDKs) for iOS and Android devices. The SDK empowers developers to integrate mobile device barcode recognition into existing apps, or to build apps based on the sample code included with the kit.
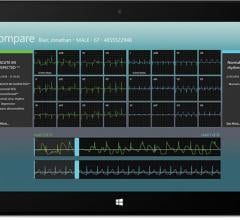
AirStrip announced the launch of AirStrip One Cardiology for all hardware using the Windows 8.1 operating system. This includes the Microsoft Surface tablet and a variety of other mobile devices, as well as laptops and desktops.
The U.S. Food and Drug Administration (FDA) cleared Admedus’s CardioCel, a tissue product to repair and treat a range of cardiovascular and vascular defects. The company is looking to complement its existing product launch in Europe with preparation for initial sales in the United States.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
The University of Michigan’s Center for Integrative Research in Critical Care (MCIRCC) has entered into a letter of intent with AirStrip with the goal of executing future collaborations, and MedStar Health has entered into a collaboration agreement with AirStrip. Both agreements are part of the AirStrip Innovation Marketplace (AIM) Program.
Scripps Green Hospital has become the first hospital in the United States to implant the world's smallest implantable cardiac monitoring device. Scripps Clinic cardiologist John Rogers, M.D., successfully completed the first implant of the Reveal Linq Insertable Cardiac Monitor (ICM) in 71-year-old San Diego resident Chuck Beal.
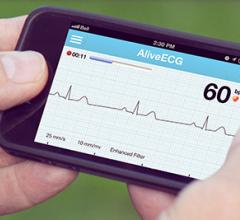
AliveCor Inc. announced a partnership with Practice Fusion, a physician-patient community. Physician offices will now be able to import the AliveCor Heart Monitor’s ECG readings, annotated reports and reviews into the Practice Fusion HER. It an attempt to allow them to see a more complete picture of their patients’ medical history and make more educated treatment decisions. Practice Fusion's EHR is used by more than 100,000 medical professionals on a monthly basis, and all of whom will now be offered the ability to import AliveCor ECGs directly into their EHR. Through the AliveECG app physicians can record a patient’s ECG, obtain an expert review, annotate and electronically transfer this data into the EHR within seconds.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Cordis Corp. announced an agreement with TriReme Medical Inc. that grants the company exclusive distribution rights for the Chocolate PTA Balloon Catheter.
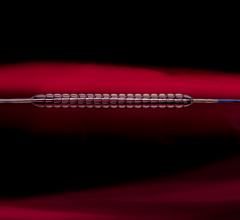
The U.S. Food and Drug Administration (FDA) approved Biosense Webster Inc.’s Thermocool Smarttouch Catheter. The therapeutic catheter enables direct and real-time measurement of contact force during catheter ablation procedures for patients suffering from drug-resistant paroxysmal atrial fibrillation (Afib), sustained monomorphic ischemic ventricular tachycardia and Type I atrial flutter.
Determining the appropriate use of cardiovascular imaging requires analyzing the “complex interplay” among care processes and their quality, patient health outcomes and medical costs, according to a health policy statement released by the American College of Cardiology and endorsed by 14 other relevant medical societies.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
February 28, 2014 — Lexmark’s Perceptive Software demonstrated its Universal Clinical Platform solution for enterprise clinical content management at the 2014 meeting of the Healthcare Information and Management Systems Society (HIMSS).

There is now a 3-D elastic membrane made of a soft, flexible, silicon material that is precisely shaped to match the heart's epicardium. Current technology is two-dimensional and cannot cover the full surface of the epicardium or maintain reliable contact for continual use without sutures or adhesives. The 3-D device was created by an international team of biomedical engineers and materials scientists and Igor Efimov, Ph.D., at the School of Engineering and Applied Science at Washington University in St. Louis.
February 28, 2014 — Mercator MedSystems released pilot study data from a single-arm, open label study of 20 patients suffering with peripheral artery disease (PAD), which reveal sustained, positive clinical outcomes from baseline to 12 months and a one-year primary patency rate of 83 percent.
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...

February 28, 2014 — Igor Efimov, Ph.D., from the School of Engineering & Applied Science at Washington University in St. Louis, and an international team of biomedical engineers and materials scientists have created a 3-D elastic membrane made of a soft, flexible, silicon material that is precisely shaped to match the heart's epicardium, or the outer layer of the wall of the heart.
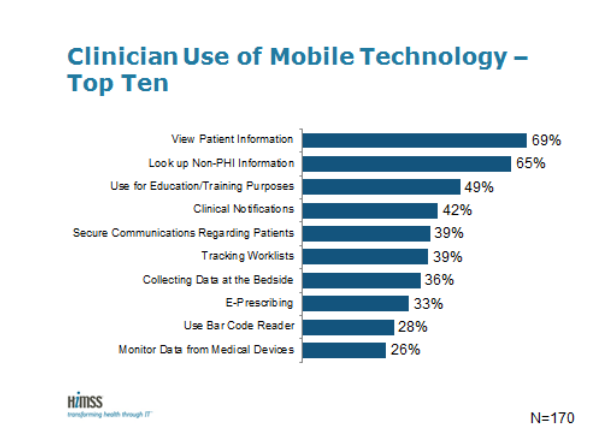
HIMSS Analytics published the results of the third Annual HIMSS Analytics Mobile Survey at its annual conference in Orland, Fla. The survey examines use of mobile devices in provider patient care improvement initiatives. For the first time this year, the survey questions were modified to closely align with the six areas of the mHIMSS Roadmap, a strategic framework for providers to implement mobile and wireless technologies.
The U.S. Food and Drug Administration (FDA) cleared IMRIS Inc.’s upgraded Visius Surgical Theatre, which integrates Siemens' high-field MR scanners. The core imaging technology based on Siemens Aera 1.5T(tesla) and Skyra 3.0T technology helps IMRIS deliver better image quality with higher signal-to-noise ratio. It also provides faster 3-D image acquisition and improved ease-of-use and workflow during neurosurgical procedures using intraoperative MRI (iMRI).


 March 03, 2014
March 03, 2014