The increasing number of percutaneous coronary interventions (PCIs) being performed at low-volume centers without on-site cardiac surgery backup has driven the need for new safety and quality protocols. This is according to an expert consensus document written by a committee representing the Society for Cardiovascular Angiography and Interventions (SCAI), the American College of Cardiology Foundation (ACCF) and the American Heart Association (AHA). The document outlines steps hospitals can take to provide the safest possible environment for PCI when the facility does not provide cardiac surgery as a backup should complications occur.
Generating new cardiac muscle from human embryonic stem cells (hESCs) and/or induced pluripotent stem cells (iPSC) could fulfill the demand for therapeutic applications and drug testing. The production of a similar population of these cells remains a major limitation, but in a study just published in Stem Cells Translational Medicine, researchers now believe they have found a way to do this.
Volumes are high at the Bryan Medical Center/Bryan Heart Hospital, located in Lincoln, Neb., resulting in large amounts of data. Managing and monitoring all this data is critical, so the center turned to Lumedx Analytics performance management software. The new solution, staff says, has turned out to be both versatile and powerful.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
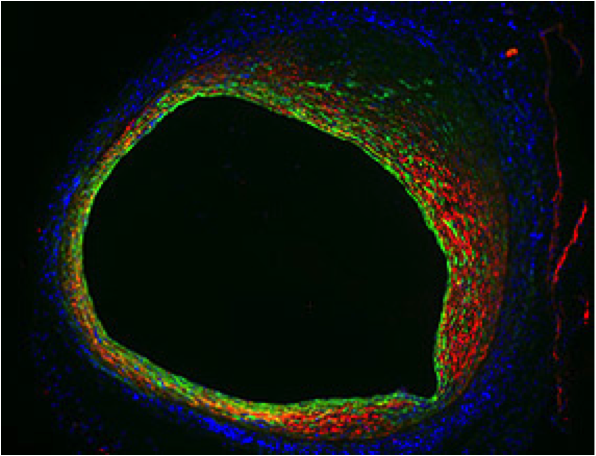
National Institutes of Health researchers have identified a biological pathway that contributes to the high rate of vein graft failure following bypass surgery. Using mouse models of bypass surgery, they showed that excess signaling via the transforming growth factor beta (TGF-Beta) family causes the inner walls of the vein become too thick. This slows down or sometimes even blocks the blood flow that the graft was intended to restore. Inhibition of the TGF-B signaling pathway reduced overgrowth in the grafted veins.
Boston Scientific Corp. received CE marking for the Rebel Platinum Chromium Coronary Stent System, the company's latest generation bare metal stent for the treatment of coronary artery disease (CAD).
Janssen Research & Development LLC (Janssen) announced the U.S. Food and Drug Administration (FDA) issued complete response letters (CRLs) regarding supplemental New Drug Applications (sNDAs) for the use of rivaroxaban, an oral anticoagulant, to reduce the risk of secondary cardiovascular events — defined as heart attack, stroke or death — in patients with acute coronary syndrome (ACS) and to reduce the risk of stent thrombosis in the same population, in combination with standard antiplatelet therapy.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Privately held On-X Life Technologies Inc. (On-X LTI) launched its European marketing campaign for the On-X Plus 1.5 Aortic Heart Valve in concert with its Great Britain distributor Vascutek Corp. at the Annual Meeting & Cardiothoracic Forum of the Society for Cardiothoracic Surgery in Great Britain & Ireland, March 10-12, Edinburgh, Scotland.
Micell Technologies Inc. announced that imaging and clinical results from the DESSOLVE I and DESSOLVE II trials of its MiStent Sirolimus Eluting Absorbable Polymer Coronary Stent System (MiStent SES) were presented at the 25th Annual Transcatheter Cardiovascular Therapeutics (TCT) Conference held in San Francisco, Oct. 27 to Nov. 1, 2013.
Houston Healthcare is putting patient safety first by offering low dose computed tomography (CT) exams with the industry’s best solutions and a comprehensive dose management approach from Toshiba America Medical Systems Inc.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
iRhythm Technologies Inc., a healthcare information services company, announced that new study data support the use of its ZIO Service to help identify underlying cardiac arrhythmias in patients who have had a stroke or transient ischemic attack (TIA).
The U.S. Food and Drug Administration (FDA) cleared Toshiba’s CT Myocardial Perfusion capability. Available on Toshiba’s Aquilion One and Aquilion OneVison Edition CT systems, Myocardial Perfusion allows clinicians to visualize myocardial ischemia with CT, providing a clinical and operational solution to make work flow.
McKesson announced a cardiology solution called Qualitative Intelligence and Communication System (QICS). QICS for cardiology helps organizations automate and manage cardiology workflows to promote efficiency and effectiveness. The solution offers integrated workflows and results communications management within the cardiovascular information system (CVIS).
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
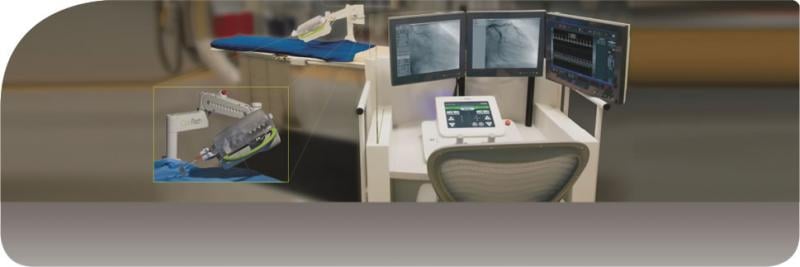
For patients living in rural areas, life-saving procedures such as an angioplasty may only be available at a facility more than 200 miles away. To bridge this distance, Corindus Vascular Robotics is partnering with Sanford Health and The Leona M. and Harry B. Helmsley Charitable Trust to launch a feasibility study for a remote robotic systems cath lab telecardiology program.
Countries across the globe are struggling to combat issues such as escalating healthcare costs, poor or inconsistent quality of healthcare, rapid expansion in healthcare insurance and changing healthcare reform mandates. Growing consumerism, globalization, changing demographics and lifestyles, and growing incidences of diseases that are expensive to treat further exacerbate these. Resolving these issues is a daunting task faced by healthcare stakeholders, highlighting the need for proactive, collaborative and systemic models. Several initiatives and healthcare reforms have been developed in order to support the adoption and implementation of clinical decision support software (CDSS) solutions.
The U.S. Food and Drug Administration (FDA) cleared Cardiovascular Systems Inc.’s Diamondback 360 60 cm Peripheral Orbital Atherectomy Systems (OAS) for the treatment of peripheral arterial disease (PAD).

 March 18, 2014
March 18, 2014