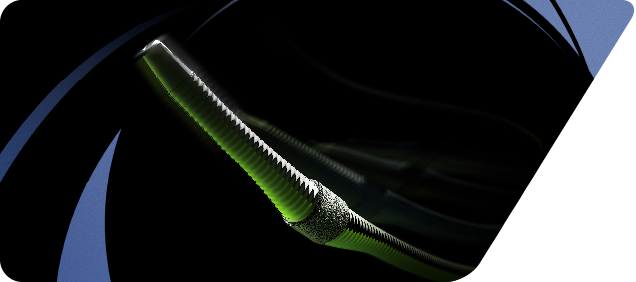
March 11, 2014 — The U.S. Food and Drug Administration (FDA) cleared Cardiovascular Systems Inc.’s Diamondback 360 60 cm Peripheral Orbital Atherectomy Systems (OAS) for the treatment of peripheral arterial disease (PAD).
The 60 cm Diamondback device is compatible with 4 French introducer sheaths and is available in two crown designs: the 1.25 mm Micro Crown and the 1.25 mm Solid Crown. Each device offers a short shaft length, a small profile and a flexible shaft.
The Diamondback 360 Peripheral Orbital Atherectomy Systems are percutaneous orbital atherectomy systems indicated for use as therapy in patients with occlusive atherosclerotic disease in peripheral arteries and stenotic material from artificial arteriovenous dialysis fistulae. The systems are contraindicated for use in coronary arteries, bypass grafts, stents or where thrombus or dissections are present. Although the incidence of adverse events is rare, potential events that can occur with atherectomy include: pain, hypotension, CVA/TIA, death, dissection, perforation, distal embolization, thrombus formation, hematuria, abrupt or acute vessel closure, or arterial spasm.
For more information: www.csi360.com


 September 12, 2025
September 12, 2025 









