BioTelemetry Inc. entered into a definitive agreement to acquire the cardiac patient services business of Biomedical Systems Corp. (BMS) for aggregate consideration of $8.65 million at closing.
Sorin Group has purchased Oscor Inc. lead business, including a lead manufacturing facility in the Dominican Republic for a value of approximately $20 million.
New Vinyl Blood Pressure Cuffs from SunTech Medical deliver accurate blood pressure (BP) readings while providing an economical alternative to traditional durable blood pressure cuffs. Designed for short-term use, these easily cleaned cuffs work with automated BP monitors in clinical areas such as hospital emergency rooms and EMS transport where disinfection is critical.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...

Doctors at the Stony Brook Heart Institute Electrophysiology Lab are using a new nonsurgical technique called the Lariat Suture Delivery Device to treat patients with atrial fibrillation who cannot tolerate blood-thinning medication.
ScImage Inc. has partnered with Cedaron Medical Inc. to deliver CardiacCare with ScImage’s enterprise picture archive and communications system (PACS), PICOM365.
Boston Scientific Corp. received CE marking and launched in Europe the Ingevity family of magnetic resonance imaging (MRI) compatible pacing leads. The Ingevity family is a set of leads that can be placed using a 6 French introducer, including passive and active fixation models. Ingevity MRI pacing leads are part of the ImageReady MR-conditional pacemaker system, which includes Vitalio MRI, Formio MRI, Advantio MRI and Ingenio MRI pulse generators. When used with the Latitude NXT patient management system, these devices wirelessly monitor patients for conditions such as atrial arrhythmias.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
AccessClosure plans to launch the Mynx Ace Vascular Closure Device at the 2014 American College of Cardiology meeting, March 29-31. It is an upgrade of the company’s Mynx mechanical vascular closure product. It is designed to provide patient comfort and uses an easy-to-use deployment system to seal femoral artery access sites. The device helps reduce time to hemostasis and ambulation in patients who have undergone diagnostic or interventional endovascular procedures.
Today, PinnacleHealth is home to one of the foremost transcatheter aortic valve replacement (TAVR) programs in the world. But in the program’s infancy, PinnacleHealth needed to develop relationships between clinicians and facilitate their collaboration using the right diagnostic imaging technology to ensure success. Using a multidisciplinary, collaborative commitment from clinicians and a state-of-the-art hybrid OR lab anchored by Toshiba’s Infinix-i cardiovascular imaging system, PinnacleHealth is improving outcomes for some of its sickest patients. As one of the finest TAVR programs available today, it is also achieving fewer readmissions and lowering costs.
Surgeons in France have successfully replaced the aortic valve in two patients without opening the chest during surgery. The procedure, using totally endoscopic aortic valve replacement (TEAVR), shows potential for improving quality of life of heart patients by offering significantly reduced chest trauma. It is described in The Journal of Thoracic and Cardiovascular Surgery.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
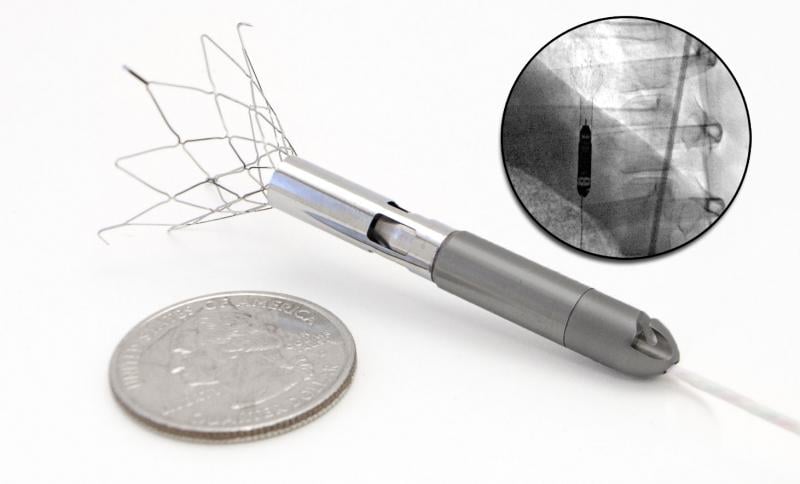
Percutaneous ventricular assist (pVAD) devices offer more hemodynamic support than the 40-year-old gold standard of intra-aortic balloon pumps (IABPs), but their cost and complexity has limited their use to all but the sickest patients. However, the quest for improving patient outcomes and new applications of pVADs for right ventricular support and heart failure indications continues to expand interest in these devices. Several of these new technologies were highlighted in sessions during the 2013 Transcatheter Cardiovascular Therapeutics (TCT) last fall.
I have watched a trend in medical technology grow from a small ripple a couple years ago into what I expect will be a tidal wave in 2014 due to growing concern over patient radiation dose levels from medical imaging. Use of radiation dose monitoring software came to the forefront when California, followed by Texas, created laws requiring medical facilities to record the amount of exposure patients receive from things like computed tomography (of which cardiac exams are among the highest dose levels used) and angiography. Adoption of this software will be further accelerated by new Joint Commission standards released in December 2013, which starting in mid-2014 requires the use of radiation dose monitoring software as part of its accreditation.
St. Jude Medical Inc. announced the first enrollments in the company’s LEADLESS Pacemaker Observational Study evaluating the Nanostim leadless pacing technology. The Nanostim pacemaker received CE marking in 2013, and post-approval implants have occurred in the United Kingdom, Germany, Italy, Czech Republic, France, Spain and the Netherlands.
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
Lumedx Corp. is a provider of vendor-neutral cardiovascular imaging and information systems (CVIS). It announced it has contracted to provide Phoebe Putney Memorial Hospital with a comprehensive CVIS that will integrate patient data and images across five different sites. The Lumedx CVIS will consolidate data, improve physician access to patient information, streamline clinical workflows, and support outcomes analysis and registry reporting for the Phoebe Heart and Vascular Center.
Thoratec Corp. initiated a voluntary worldwide Medical Device Correction in order to update its labeling and training materials for the HeartMate II LVAS Pocket System Controller. The following information is provided as a reinforcement of the initial release.
Patients with coronary artery disease who received an Endeavor or Resolute drug-eluting stent from Medtronic Inc. and subsequently interrupted their dual antiplatelet therapy (DAPT) earlier than current guidelines recommend experienced no increased risk of stent thrombosis at one year or more of follow up. Both devices elute the drug zotarolimus to reduce the risk of restenosis. This is according to findings from several studies presented at the 25th annual Transcatheter Cardiovascular Therapeutics (TCT) scientific symposium.

 March 24, 2014
March 24, 2014