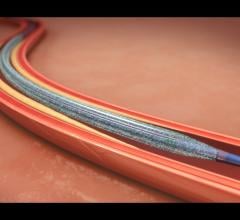
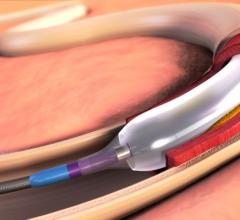
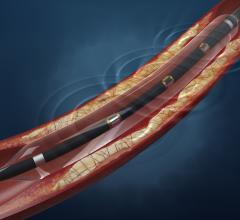
In 2015, the FDA approved the first two drug-coated balloons for the U.S. market to treat peripheral artery disease in ...
Peripheral Artery Disease (PAD)
This channel includes news, interventions, and new technology innovations for peripheral artery diease, PAD and critical limb ischemia.
March 9, 2015 — Boston Scientific Corp. announced the settlement of the breach of merger agreement lawsuit brought by ...
February 23, 2015 – Biotronik announced the presentation of data from the iliac arm of the BIOFLEX-I clinical trial at ...
March 6, 2015 — Merck announced results from two post-hoc analyses of the TRA 2°P TIMI 50 (Thrombin Receptor Antagonist ...

March 2, 2015 — Cardinal Health announced plans to acquire Johnson & Johnson’s Cordis business, a leading global ...
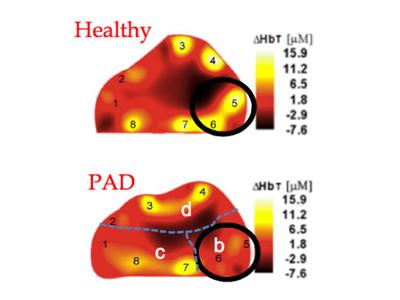
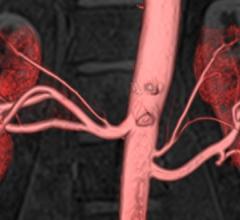
February 26, 2015 — Approximately 8 to 12 million people in the United States alone are suffering from peripheral ...
February 25, 2015 — CBSET, a not-for-profit preclinical research institute, announced that its scientists have defined ...
February 23, 2015 — Medtronic announced that the U.S. Centers for Medicare and Medicaid Services (CMS) has approved a ...
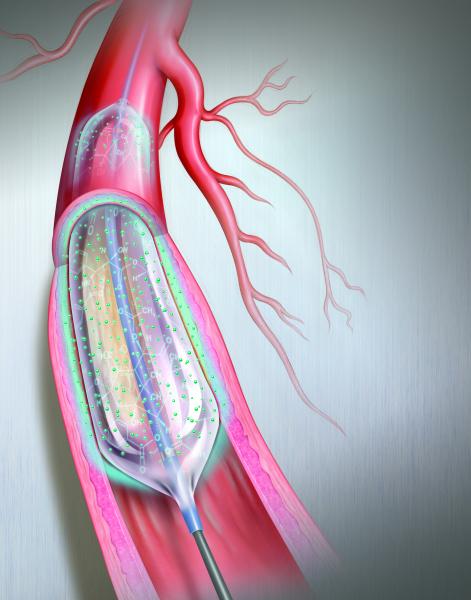
Drug-eluting balloons (DEBs), also referred to as drug-coated balloons (DCBs), have been one of the long-awaited new ...
February 9, 2015 — Ensuring that patients with peripheral arterial disease (PAD) get tested and treated with minimally ...
February 6, 2015 — United States hospitals began using a new medical device from Medtronic plc called the In.Pact ...
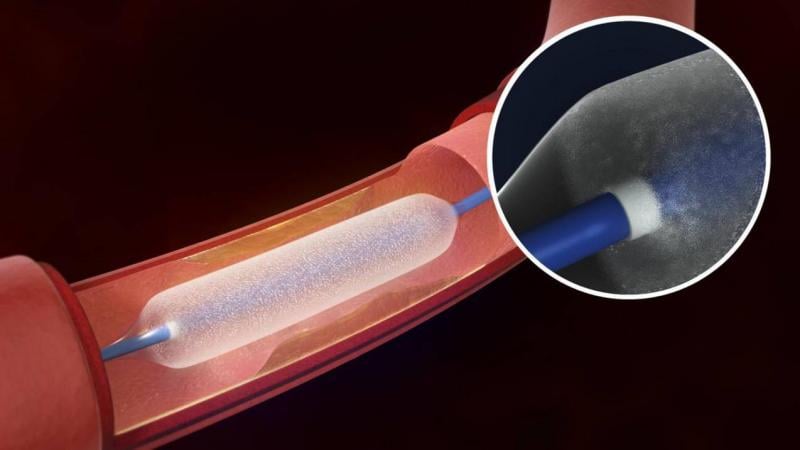
January 30, 2015 — ECRI Institute has created a report that offers an overview of drug-eluting balloon (DEB) technology ...
January 16, 2015 — Non-contrast enhanced MR angiography (MRA) techniques have attracted interest in the medical ...
January 13, 2015 — Shockwave Medical announced CE Mark regulatory approval for the company’s Lithoplasty balloon ...
January 9, 2015 — Covidien announced it has received CE Mark approval for its Stellarex drug-coated angioplasty balloon ...


 April 02, 2015
April 02, 2015