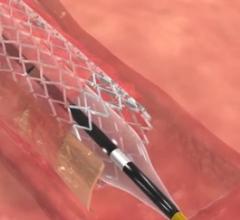
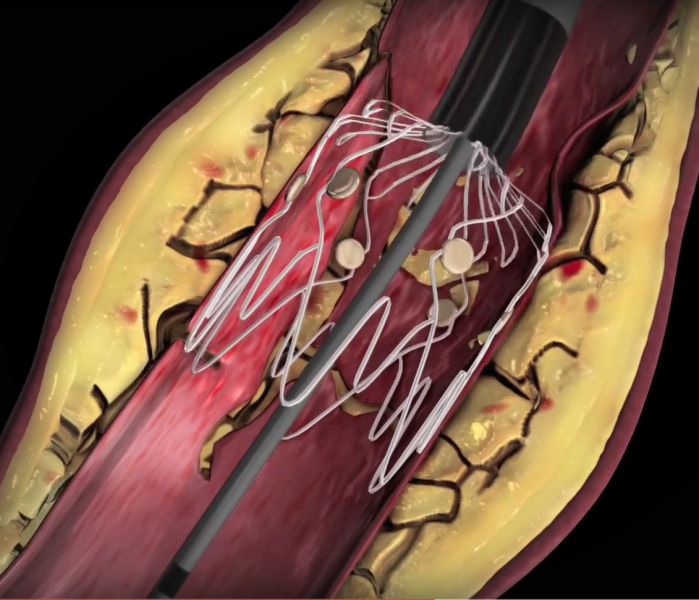
April 27, 2016 — Veniti Inc. announced the first successful treatment with the Vici Verto Venous Stent System of a ...
Peripheral Artery Disease (PAD)
This channel includes news, interventions, and new technology innovations for peripheral artery diease, PAD and critical limb ischemia.
April 18, 2016 — The American College of Radiology (ACR), as a member of a coalition of leading medical societies ...
March 29, 2016 — Corindus Vascular Robotics Inc. announced that the U.S. Food and Drug Administration (FDA) has cleared ...
March 28, 2016 — NuCryo Vascular LLC announced the launch of the Next Generation Cryoplasty Inflation device. The device ...
March 22, 2016 — PinnacleHealth CardioVascular Institute enrolled the first patient in Pennsylvania into the TOBA II ...
March 17, 2016 — Boston Scientific announced in late February that the Eluvia Drug-Eluting Vascular Stent System ...
March 16, 2016 — Bioengineers and physicians at the University of California, San Diego have developed a potential new ...
March 3, 2016 — Avinger Inc. announced the company has received 510(k) clearance from the U.S. Food and Drug ...
It is estimated that more than 10 million people in the United States are affected by peripheral arterial disease (PAD) ...
February 19, 2016 — Peripheral stents will account for over $4.6 billion in worldwide sales by 2020, according to a new ...
February 17, 2016 — Biotronik announced a partnership with Maquet Medical Systems USA to distribute Biotronik peripheral ...
February 8, 2016 — Stentys announced the first distribution agreements for its drug-eluting stent for treating below-the ...
January 29, 2016 — Intact Vascular Inc. announced that positive six-month results from its Tack Optimized Balloon ...
January 28, 2016 — A new imaging technique could reduce the need for amputation in patients with critical limb ischemia ...
January 15, 2016 — Biointegrated sensors for long-term, continuous tracking of body chemistry may make health and ...

 April 27, 2016
April 27, 2016