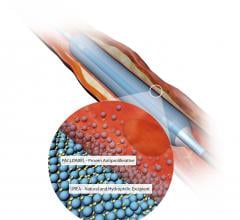
April 26, 2017 — Boston Scientific announced results from the RANGER SFA trial for the Ranger Paclitaxel-Coated PTA Ball ...
Peripheral Artery Disease (PAD)
This channel includes news, interventions, and new technology innovations for peripheral artery diease, PAD and critical limb ischemia.
April 12, 2017 — PinnacleHealth CardioVascular Institute last week enrolled the first patient in the United States in a ...
March 16, 2017 — W. L. Gore & Associates Inc. recently announced the Health Canada approval of the Gore Tigris Vascular ...
March 9, 2017 — Medtronic plc announced the launch of the IN.PACT BTK study to evaluate the effectiveness of a drug ...
March 2, 2017 — Intact Vascular Inc. announced in February that its Tack Optimized Balloon Angioplasty II Below the Knee ...
February 15, 2017 — Mercator MedSystems announced that the national co-principal investigators of the company’s DANCE tr ...
February 8, 2017 — Cardiovascular Systems (CSI) presented six-month data from its LIBERTY 360° post-market study in a ...
February 3, 2017 — Avinger Inc. recently announced positive two-year clinical data from the pivotal VISION study of the ...
January 31, 2017 — W. L. Gore & Associates (Gore) announced that the Gore Viabahn VBX Balloon Expandable Endoprosthesis ...
January 31, 2017 — Bard Peripheral Vascular Inc. is recalling the Halo One Thin-Walled Guiding Sheath because the sheath ...
January 27, 2017 — HyperMed Imaging Inc. announced that it has received clearance from the U.S. Food and Drug ...
January 11, 2017 — Avinger Inc. recently announced the U.S. launch of an enhanced version of the company’s Lightbox ...
January 5, 2017 — A new report published by Allied Market Research forecasts that the global thrombectomy devices market ...
December 23, 2016 — The U.S. Food and Drug Administration (FDA) granted a new 510(k) clearance for Avinger’s Lightbox ...
December 13, 2016 — Biotronik announced the presentation of data confirming the efficacy of the Pulsar-18 bare metal ...

 April 26, 2017
April 26, 2017