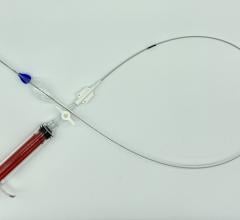
January 31, 2017 — W. L. Gore & Associates (Gore) announced that the Gore Viabahn VBX Balloon Expandable Endoprosthesis (VBX Stent Graft) has received U.S. Food & Drug Administration (FDA) approval for treatment of de novo or restenotic lesions found in iliac arteries. The approval includes lesions at the aortic bifurcation. This marks the availability of the only balloon expandable stent graft with an indication for the iliac artery.
“The VBX Stent Graft demonstrated notable immediate and nine-month safety and efficacy in treating patients with iliac occlusive disease which can be attributed to the exceptional device design,” said Jean Bismuth, M.D.
Bismuth continued, “Overall, there were multiple clinical benefits observed, including no median change in the device length upon deployment, and a 100 percent technical success rate with no occurrences of stent dislodgement or significant residual stenosis. The study device performed well in disadvantaged lesions, including occlusions, which speaks to its trackability, radial strength, conformability, and stent retention.”
Of the patients in the Gore VBX FLEX IDE clinical study (n=134), 32 percent presented with TASC II type C or D lesions, 18 percent required contralateral access to the lesion, and 42 percent involved kissing stents at the aortic bifurcation. Clinical data from the study conducted for FDA approval reflected that the design components of the VBX Stent Graft were resilient both during stenting procedures and over time:
- 100 percent success rate in device delivery and coverage of target lesions in all study subjects;
- 100 percent success rate in reducing the target lesion to less than or equal to 30 percent of the original stenosis;
- Zero change in median length of the device upon deployment; and
- 96.9 percent primary patency at nine-months, including a 95.3 percent primary patency rate in those patients with TASC II C or D type lesions.
Further, there were no reported incidences of device dislodgement, failures in stent integrity or device-related serious adverse events through the primary endpoint follow-up, meaning no additional costs incurred for either endovascular or surgical stent removal. The VBX Stent Graft does not require pre-dilation, which reduces the number of balloons required, and the longer lengths available reduce the need to use multiple stents for extensive lesions, both of which also contribute to procedural cost savings.
The VBX Stent Graft was developed utilizing the small diameter, ePTFE stent graft technology from the Gore Viabahn Endoprosthesis. The VBX Stent Graft is available in a range of diameters from 5 to 11 millimeters and lengths of 15, 19, 29, 39, 59 and 79 millimeters to cover a wide variety of treatment needs.
For more information: www.goremedical.com


 September 18, 2025
September 18, 2025