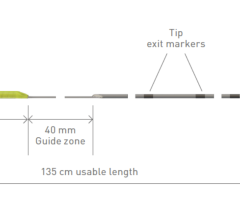
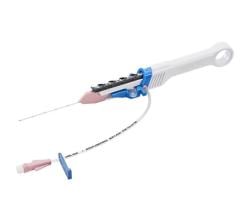
Image courtesy of Roxwood Medical
December 31, 2014 — Roxwood Medical Inc. announced U.S. Food and Drug Administration (FDA) clearance for the U.S. commercialization of its CenterCross Catheter for use in the coronary and peripheral vasculature.
The CenterCross Catheter is designed to be used in conjunction with guidewires and microcatheters to access discrete regions of the coronary and peripheral vasculature and enable clinicians to better address wire-crossing of complex lesions. The catheter incorporates a unique self-expanding scaffold to stabilize off-the-shelf interventional tools, such as guidewires and microcatheters, in the center of the artery near the target lesion.
Initial cases were performed by some of the leading interventional cardiologists across the United States.
For more information: www.roxwoodmedical.com


 January 29, 2026
January 29, 2026