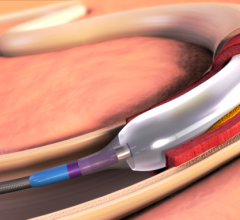
October 31, 2014 — Metro Health (Michigan) cardiovascular specialist Jihad Mustapha, M.D., is one of the first ...
Peripheral Artery Disease (PAD)
This channel includes news, interventions, and new technology innovations for peripheral artery diease, PAD and critical limb ischemia.
Oct. 27, 2014 — The U.S Food and Drug Administration (FDA) cleared the EverFlex Self-Expanding Peripheral Stent System ...
October 23, 2014 — The use of stents has improved management and outcomes of coronary artery disease, and clinical ...
October 14, 2014 — Presented for the first time at the 2014 Transcatheter Cardiovascular Therapeutics (TCT) conference ...
October 8, 2014 — More than 40 percent of Americans aged 40 and older have experienced one or more of the most common ...
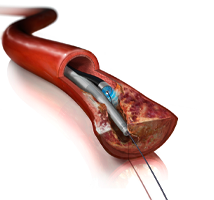
September 26, 2014 — The first large prospective study to examine the effectiveness of laser atherectomy in the ...
September 9, 2014 — Cook Medical has enrolled the first patient in the clinical study in China of its Zilver PTX Drug ...

September 8, 2014 — Directional atherectomy is safe and effective as a frontline therapy for the treatment of peripheral ...

August 28, 2014 — Renal artery stenting to open blockages in the kidney arteries may benefit patients who have ...
August 12, 2014 — Teleflex Inc.’s subsidiary Hotspur Technologies Inc. received U.S. Food and Drug Administration (FDA) ...
August 8, 2014 — Biotronik announced the completion of patient enrollment in the superficial femoral artery (SFA) arm of ...
July 29, 2014 — Biotronik announced that the first patient has been enrolled in the investigator-initiated BIOLUX 4EVER ...
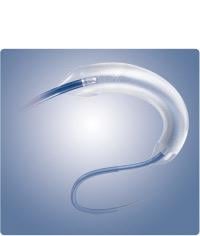
July 28, 2014 — Boston Scientific Corp. received CE mark for the Ranger Paclitaxel-coated PTA Balloon Catheter. The ...

 October 31, 2014
October 31, 2014