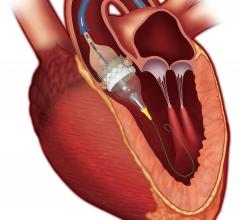
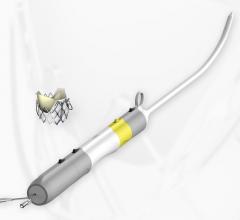
The rise cardiovascular disease has been instrumental in fueling the coronary stent market share in the past few years ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).

Many of the latest advances in cardiovascular imaging technologies are unveiled each year at the Radiological Society of ...
January 17, 2019 — Philips announced the launch of Azurion with FlexArm, designed to enhance positioning flexibility for ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
January 17, 2019 — The U.S. Food and Drug Administration (FDA) issued a letter Jan. 17, 2019, to healthcare providers ...
January 16, 2019 — Cerenovus, of Johnson & Johnson Medical Devices Companies, recently launched the EXCELLENT Registry ...
January 16, 2019 — Shockwave Medical Inc. has initiated its U.S. Food and Drug Administration (FDA) Investigational ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
January 16, 2019 — Abbott announced the U.S. Food and Drug Administration (FDA) approved the Amplatzer Piccolo Occluder ...
January 15, 2019 — Boston Scientific Corp. and Edwards Lifesciences Corp. announced that the companies have reached an ...
Mitral regurgitation (MR) is one of the most common types of heart valve diseases in the United States, affecting ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...

Here are a few of the takeaways from the clinical studies presented and new technology shown on the exhibit floor at the ...
Mark Anderson, M.D., FACS, vice chair of cardiac surgery services and cardiothoracic surgeon at Hackensack University ...
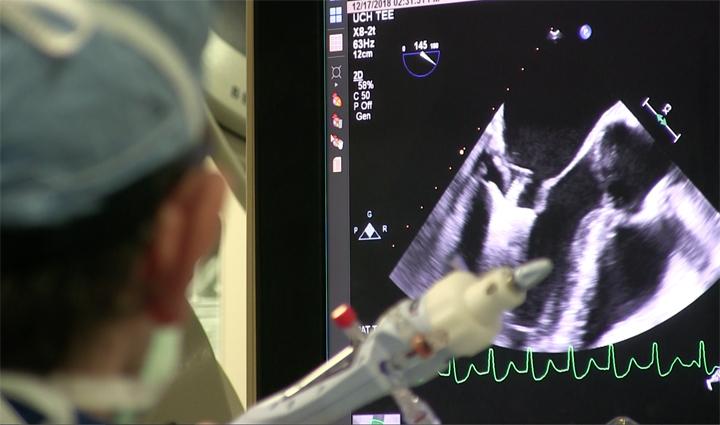
Robert Quaife, M.D., director of advanced cardiac imaging, University of Colorado Hospital, explains why advanced ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
January 8, 2019 — The Society of Cardiovascular Computed Tomography (SCCT) has released a new expert consensus document ...
January 7, 2019 — JC Medical announced the successful treatment of the first U.S. patient with the company’s ...
Karen Orjuela, M.D., assistant professor of neurology at the University of Colorado Stroke and Brain Aneurysm Center, ex ...

 January 22, 2019
January 22, 2019