August 8, 2018 — Boston Scientific Corp. announced it has signed an agreement to acquire Veniti Inc., which has developed and commercialized the Vici Venous Stent System for treating venous obstructive disease. Boston Scientific has been an investor in Veniti since 2016 and currently owns 25 percent of the company. The transaction price for the remaining stake consists of $108 million up-front cash, as well as up to $52 million in payments contingent upon U.S. Food and Drug Administration (FDA) approval of the Vici stent system.
Venous obstructive disease — instances of abnormal, blocked or damaged veins — affects more than 1.1 million people in the United States and Western Europe annually. Vein obstructions — often caused by conditions such as deep vein thrombosis, post thrombotic syndrome and May-Thurner syndrome — can prevent proper blood circulation and cause patients to experience pain, swelling, ulcers and a diminished quality of life. Physicians often choose to open the obstructed vessel with a stent to reinstate proper blood flow to the heart and lungs and reduce a patient's symptoms.
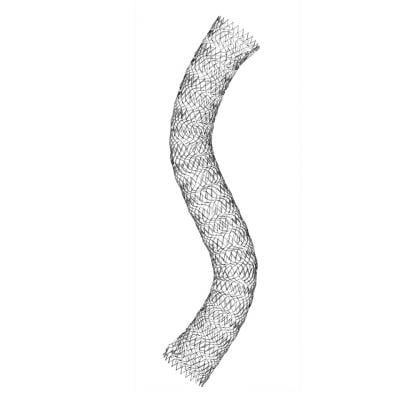
The self-expanding, nitinol Vici stent system was developed specifically for use in the venous anatomy, which presents different challenges than placing stents in the arterial vascular system. The stent is designed to withstand compression and maintain patency and flexibility over the course of a patient's life expectancy.
The Vici stent system received CE Mark in 2013 and Veniti submitted a pre-market approval (PMA) application to the FDA in June, leveraging results from the recently completed VIRTUS pivotal study. Currently in the U.S., there are no stent technologies specifically indicated for use in the peripheral venous system.
In the U.S., the VICI Stent System is an investigational device and is not available for sale.
For more information: www.bostonscientific.com, www.veniti.com


 November 14, 2025
November 14, 2025 









