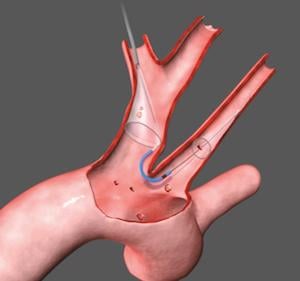
August 3, 2018 — Boston Scientific Corp. announced it has recently closed its acquisition of Claret Medical Inc., a privately-held company that developed and commercialized the Sentinel Cerebral Embolic Protection System. The device is U.S. Food and Drug Administration (FDA)-cleared to protect patients against the risk of stroke in transcatheter aortic valve replacement (TAVR) procedures.
Boston Scientific also announced that the U.S. Centers for Medicare and Medicaid Services (CMS) granted a New Technology Add-on Payment (NTAP) designation for the Sentinel system.
The NTAP designation was granted as part of the federal fiscal year 2019 Inpatient Prospective Payment System (IPPS). The NTAP designation, awarded to new medical devices determined to substantially improve the diagnosis or treatment of Medicare beneficiaries, will be effective on Oct. 1, 2018.
The Sentinel System – which received CE Mark in 2014 and FDA clearance in 2017 – is the only device commercially available used to protect patients against the risk of stroke during TAVR, a minimally-invasive procedure to replace the aortic valve in patients with severe aortic stenosis. Embolic debris such as calcium or tissue can break loose during the procedure, travel through the bloodstream towards the brain and potentially cause neurological and neurocognitive damage. Recent studies have estimated approximately four percent of patients experience a clinically-apparent stroke within 30 days of a TAVR procedure.1,2,3,4,5,6 The landmark SENTINEL trial, which led to regulatory clearance, demonstrated that the Sentinel System reduced the incidence of strokes by 63 percent within the first 72 hours of the procedure.7
Boston Scientific announced a definitive agreement to acquire Claret Medical on July 20, 2018 for $220 million in up-front cash with an additional $50 million payment for reaching a reimbursement-based milestone, which has been fulfilled with the recent NTAP designation.
For more information: www.bostonscientific.com
References
1. Leon, et al., N Engl J Med. 2010;363:1597-1607.
2. Webb, et al., J Am Coll Cardiol Intv. 2015;8:1797-1806.
3. Smith, et al., N Engl J Med. 2011;364:2187-98.
4. Leon, et al., N Engl J Med 2016;374:1609-20.
5. Popma, et al., J Am Coll Cardiol 2014;63:1972-81.
6. Adams, et al., N Engl J Med 2014;370:1790-98.
7. Kapadia S, et al. JACC. Jan 2017; 69(4): 367-377.


 November 14, 2025
November 14, 2025 









