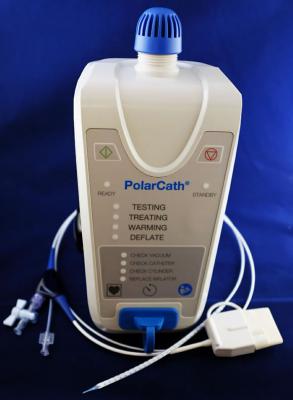
August 14, 2018 — NuCryo Vascular announced that the company has signed a commercialization agreement with Lokai Medical, a specialty distributor of coronary/peripheral and interventional devices, to distribute the PolarCath Balloon Dilatation System in the United States.
The controlled cooling of the plaque and artery wall in balloon cryoplasty provides three potential benefits: uniform vessel dilation with less vessel trauma, reduced vessel wall recoil and induction of apoptosis, which promotes the natural cell death of the smooth muscle cells that otherwise proliferate to cause restenosis. The PolarCath Cryoplasty system was invented by James Joye, M.D., an interventional cardiologist in Mountain View, Calif. who is a pioneer in the development of medical devices to treat peripheral arterial disease (PAD).
“I am very excited for the next-generation reusable cryoplasty inflation device that NuCryo has re-engineered and brought back to market,” said Jonathan Aliota, M.D., interventional cardiologist from Houston. “As a previous cryoplasty user with Boston Scientific, I recently reincorporated it back into my peripheral treatment algorithm and have been extremely happy with the results. In addition, the cost, ease of use and time savings as compared to other balloons on the market have been well received at my facility.”
The PolarCath Peripheral Dilatation System received U.S. Food and Drug Administration (FDA) clearance to dilate stenosis in the peripheral vasculature (iliac, femoral, popliteal, infrapopliteal, renal and subclavian arteries) and for the treatment of obstructive lesions of polytetrafluoroethylene (PTFE) access grafts or arteriovenous dialysis fistulae. The PolarCath System is also indicated for post-deployed stent expansion of self-expanding peripheral vascular stents.
For more information: www.nucryovasc.com


 June 13, 2024
June 13, 2024 








