October 31, 2011 – To ensure MR imaging is as efficient and safe as possible, Toshiba America Medical Systems has unveiled enhancements to its Vantage Titan MR (magnetic resonance) product line, including the first high-density 16-channel flexible coil system (works-in-progress). The new additions to the Vantage Titan MR systems are designed to make it easier for clinicians to complete high-quality exams and improve diagnostic efficiency.
October 31, 2011 — On Oct. 18, 2011, the TICH-BIOTRONIC Pacemaker Wireless Monitoring Services Centre was co-founded in China’s Tianjin Economic-Technological Development Area (TEDA) by TEDA International Cardiovascular Hospital (TICH) of Tianjin and Biotronik (Beijing) SE & Co. KG of Germany.
October 31, 2011 — American College of Cardiology (ACC) President David Holmes, M.D., FACC, commended a congressional panel that met recently to discuss the physician payment system. The briefing was orchestrated by Reps. Phil Roe and Allyson Schwartz.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
October 31, 2011 — A panel presentation at the American College of Surgeons' annual clinical congress detailed how the contemporary practice of vascular surgery has evolved during the Iraq and Afghanistan wars. This has resulted in superior outcomes for complex, combat-related vascular injuries, including increased rates of amputation-free survival following extremity wounds.
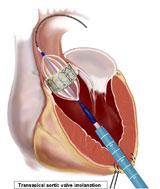
A better strategy to sell the costs of a transcatheter aortic valve implantation (TAVI) program to hospital administrators is not to base ROI on TAVI procedures, but on the increased surgical valve repair volume the program would create.

In a closely watched move – viewed as a big step toward a paradigm shift in how heart valves are repaired in the United States – a U.S. Food and Drug Administration (FDA) advisory panel in July recommended approval of the Edwards Lifesciences Sapien transcatheter heart valve.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
October 26, 2011 –Lantheus Medical Imaging announced it has been awarded three-year supplier contracts with Premier Purchasing Partners, the group purchasing unit of the Premier healthcare alliance, in the category of cardiac ultrasound contrast media for its ultrasound contrast agent, Definity Vial for (perflutren lipid microsphere) Injectable Suspension, and its magnetic resonance angiography (MRA) blood pool imaging agent, Ablavar (gadofosveset trisodium).
October 26, 2011 – Older men whose bodies produce higher levels of testosterone may be protected against heart attacks and strokes, new medical research suggests.
October 26, 2011 – St. Jude Medical announced it has received U.S. Food and Drug Administration (FDA) clearance for its Ilumien system, the first integrated diagnostic technology that combines optical coherence tomography (OCT) and fractional flow reserve (FFR) technologies on one platform. The combined system offers physicians advanced physiological and anatomical insight to improve the diagnosis and treatment of coronary artery disease.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
October 26, 2011 — Medtronic Inc. announced the start of SYMPLICITY HTN-3, the company’s United States clinical trial of the Symplicity Renal Denervation System for treatment-resistant hypertension. The first patient was enrolled at the Prairie Heart Institute at St. John’s Hospital in Springfield, Ill.

In a closely watched move viewed as a big step toward a paradigm shift in how heart valves are repaired in the United States, a U.S. Food and Drug Administration (FDA) advisory panel voted late yesterday to recommend approval of the Edwards Lifesciences Sapien transcatheter heart valve. The FDA Circulatory System Devices Panel recommended the valve specifically for the treatment of patients who are too sick to undergo the standard therapy of surgical valve replacement.
October 25, 2011 — Stentys S.A. announced the publication of the results of the APPOSITION I clinical study in the September issue of the medical journal EuroIntervention.
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
October 24, 2011 – The European Association of Echocardiography (EAE) has announced that it is working together with the American Society of Echocardiography (ASE) to issue joint recommendations on the usefulness of serial echocardiographic evaluations and the potential impact of more advanced ultrasound technologies (in particular speckle tracking echocardiography) in patients undergoing cancer therapy.
October 24, 2011 — NeoStem Inc. and Amorcyte Inc announced the closing of their previously publicized merger transaction following approval by the shareholders of both companies on Oct. 14, 2011. NeoStem closed on a financing raising $16.5 million in gross proceeds on July 22. The company intends to initiate, no later than the first quarter of 2012, a Phase 2 clinical trial for Amorcyte's lead product candidate, AMR-001, for the treatment of acute myocardial infarction (AMI).
October 24, 2011 — New study results reveal several underlying causes of non-ischemic sudden cardiac death (SCD) — those not related to coronary artery disease (CAD) — including alcohol, obesity and myocardial fibrosis. The study, published in the October edition of HeartRhythm, the official journal of the Heart Rhythm Society (HRS), also reinforces CAD as one of the most prevalent causes of SCD in the general population.


 October 31, 2011
October 31, 2011












