March 26, 2012 - New research shows that the addition of two magnetic resonance imaging (MRI) sequences to a common MR angiography technique significantly improves detection of pulmonary embolism, a potentially life-threatening condition traditionally diagnosed through computed tomography (CT). Results of the study are published online in the journal Radiology.
March 26, 2012 — Itamar-Medical unveiled its cardiovascular healthcare assessment platform, EndoPAT-MF, at the American College of Cardiology’s 61st Annual Scientific Session (ACC.12) in Chicago. The multifunction EndoPAT-MF now features five major diagnostic tools in a single system.
March 26, 2012 — GE Healthcare introduced the 510(k)-pending Discovery CT750 HD FREEdom Edition at the opening of American College of Cardiology’s 61st Annual Scientific Session in Chicago. Addressing the main challenges of cardiac imaging – coronary motion, high heart rates, calcium blooming, plaque composition and accurate myocardial perfusion – the FREEdom Edition is designed to provide a new level of cardiac computed tomography (CT) performance and to help physicians best serve patients.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
March 26, 2012 — Maquet announced positive long-term mortality results from the Balloon Pump-Assisted Coronary Intervention Study (BCIS-1), which evaluated the effect on major complications and mortality of elective use of an intra-aortic balloon pump (IABP) in patients with low ejection fraction undergoing high-risk percutaneous coronary intervention (PCI).
March 23, 2012 — New findings from a research study led by Scripps Translational Science Institute (STSI), a collaborative program between Scripps Health and the Scripps Research Institute (TSRI), shows a new blood test may be useful in helping doctors predict who is at risk for an imminent heart attack.
March 23, 2012 — Researchers using the VAP (vertical auto profile) Cholesterol Test to isolate lipid subfractions and assess a new anti-flushing agent have reported significant baseline reductions in the cholesterol content of lipoproteins across the entire VLDL to LDL density range in dyslipidemic patients.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
March 23, 2012 — Improving the ability to diagnose cardiac disease with advanced ultrasound imaging, Toshiba America Medical Systems Inc. will showcase the newest additions to its ultrasound product line, Aplio 500 and Aplio 300, at the American College of Cardiology’s annual meeting March 24-27, 2012 in Chicago. Toshiba will also be introducing new enhancements to its Aplio Artida flagship cardiac ultrasound.
March 23, 2012 — ScImage Inc. announced that all new ScImage PACS installations will now include off-site disaster recovery services free of charge.
March 22, 2012 — Infraredx Inc. announced that its TVC Imaging System for the true vessel characterization of coronary artery disease will be featured in 11 oral and poster presentations, including a panel during the Cardiovascular Innovations Educational Forum, at the American College of Cardiology Annual Scientific Session in Chicago.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
March 22, 2012 — At the 61st meeting of the American College of Cardiology in Chicago, GE Healthcare is introducing Centricity Cardio Enterprise, a new and comprehensive IT solution that offers cardiologists a single point of access to patient data, images and reports across the continuum of care while enhancing physician productivity and improving charge capture accuracy. Centricity Cardio Enterprise enables the cardiovascular care area with configurable workflow, tools and reports that can be shared across multiple facilities and organizations.
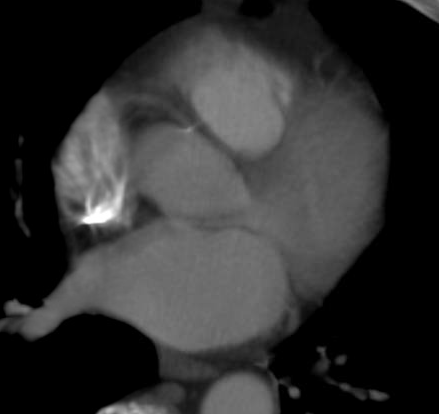
Addressing concerns over radiation from cardiac imaging and procedures, the American College of Cardiology Foundation (ACCF), Duke University Clinical Research Institute (DCRI) and American Heart Association (AHA) released recommendations to enhance radiation safety.

March 20, 2012 – The Society for Cardiovascular Angiography and Interventions (SCAI) published a first-of-its-kind paper defining best practices in the cardiac catheterization laboratory, commonly referred to as the cath lab. The paper is part of SCAI’s ongoing effort aimed to ensure the highest quality care and safety for patients undergoing interventional cardiology procedures such as angioplasty and stenting (also known as percutaneous coronary intervention or PCI). Titled “Clinical Expert Consensus Statement on Best Practices in the Cardiac Catheterization Laboratory,” the paper is e-published in Catheterization and Cardiovascular Interventions.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
March 20, 2012 - MarBlue Medical announced the global product launch of its CE-marked drug-eluting balloon (DEB) Protege as well as its CoCr stent on DEB Pioneer. The full market launch of the new DEB technology is a major step forward in Blue Medical's quest to improve the quality of life of patients with cardiovascular diseases, while at the same time reducing procedure costs.
March 20, 2012 - GE Healthcare is demonstrating its new Centricity Cardio Enterprise solution during the American College of Cardiology (ACC) 2012 meeting in Chicago. The integrated cardiovascular clinical informatics system includes both CVIS (cardiovascular information system) and CPACS (cardiovascular picture archiving and communication system) functionality to provide clinicians full access to a single, comprehensive cardiovascular patient record. Centricity Cardio Enterprise features Web-enabled technology capable of driving workflow efficiencies and integrating with your current IT systems.
March 20, 2012 - DR Systems announced new contracts with four facilities for the company's Unity CVIS (Cardiovascular Information System). The facilities are: Twin Cities Community Hospital, Good Shepherd Medical Center, HealthCare Partners Medical Group and St. Luke's Cornwall Hospital.


 March 26, 2012
March 26, 2012














