March 29, 2012 — Adding vorapaxar, an investigational platelet blocker, to standard antiplatelet therapy significantly reduces the risk of recurrent cardiovascular events in a specific group of patients, but also increased the risk of severe bleeding, including intracranial bleeding. This was according to research in the TRA 2°P-TIMI 50 Trial presented during the American College of Cardiology (ACC) 61st Annual Scientific Session in Chicago.
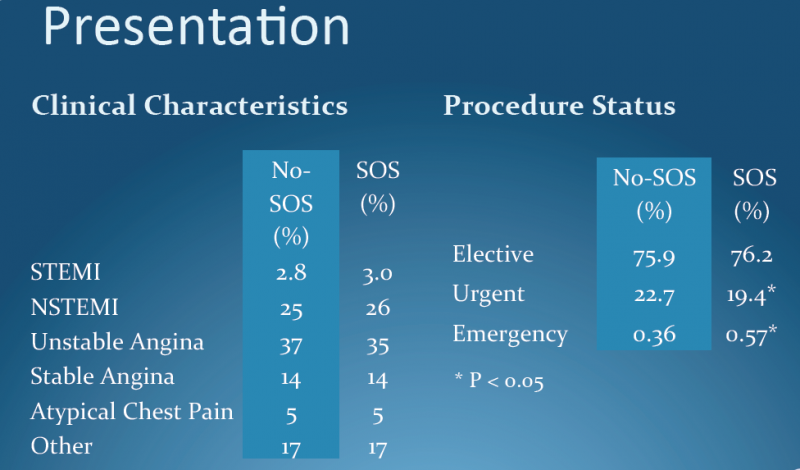
March 29, 2012 — A three-pronged intervention in Brazilian public hospitals significantly improved physician adherence to evidence-based protocols for treating acute coronary syndrome (ACS), according to data from the BRIDGE-ACS trial. The research was presented during the American College of Cardiology’s (ACC) 61st Annual Scientific Session this week in Chicago.
March 29, 2012 — VectraCor Inc. announced at the American College of Cardiology’s 61st Annual Scientific Session (ACC) in Chicago that it received U.S. Food and Drug Administration (FDA) approval for the VectraplexECG System with VectraplexAMI. It is a stand-alone cardiac monitor/electrocardiography (ECG) machine with a cardiac electric biomarker (CEB) for real-time detection of ECG changes that may be indicative of an acute myocardial infarction (AMI) plus the capability to derive a 15-lead ECG.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
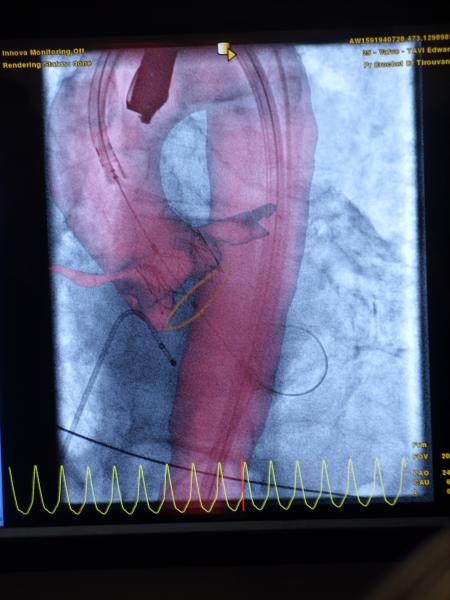
The American College of Cardiology (ACC) 2012 Scientific Session, held March 24-27 in Chicago, was the first major cardiology show this year for vendors to display their latest innovations. A couple of key trends were evident on the show floor – new technology to support trans-aortic valve replacement (TAVR) and the launch of new cardiovascular image and information systems (CVIS) to support healthcare’s proposed Stage 2 meaningful use (MU) requirements.
March 29, 2012 — St. Jude Medical Inc. announced it has received U.S. Food and Drug Administration (FDA) clearance for multiple enhancements to its PressureWire Fractional Flow Reserve (FFR) measurement guidewire. FFR measurement identifies the severity of narrowings in the coronary arteries and allows for a more effective assessment of coronary lesions, or blockages, resulting in more accurate diagnosis and improved appropriate treatment of coronary artery blockages.
March 29, 2012 — Siemens Healthcare recently expanded its solutions portfolio by offering the RaySafe i2 personal dosimetry system as an accessory for all Siemens Artis zee angiography systems. By using RaySafe i2 during imaging procedures, medical personnel obtain real-time information regarding their levels of radiation exposure, enabling them to take immediate steps to minimize exposure and establish a high-functioning radiation safety culture within their hospital.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...

Two large clinical trials were presented in the late-breaking clinical trials session at the American College of Cardiology's (ACC) 61st Annual Scientific Session this week that indicate coronary computed tomography angiography (CCTA) used as a tool to evaluate patients with chest pain in the emergency department is safe, time-efficient and cost-effective, compared to the current standard approach.
Agfa HealthCare's new modular version of its cardiovascular information system (CVIS), Impax CV12, was unveiled at the American College of Cardiology (ACC) Annual Scientific Session this week in Chicago.
GE Healthcare unveiled two recently U.S. Food and Drug Administration (FDA)-cleared angiography systems offering mobility and advanced imaging for interventional cardiology at the American College of Cardiology (ACC) 2012.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
New evidence shows that with appropriate preparation, angioplasty can be safely and effectively performed at community hospitals without on-site cardiac surgery units. This was according to data presented from the CPORT-E trial during the American College of Cardiology's (ACC) 61st Annual Scientific Session this week in Chicago.
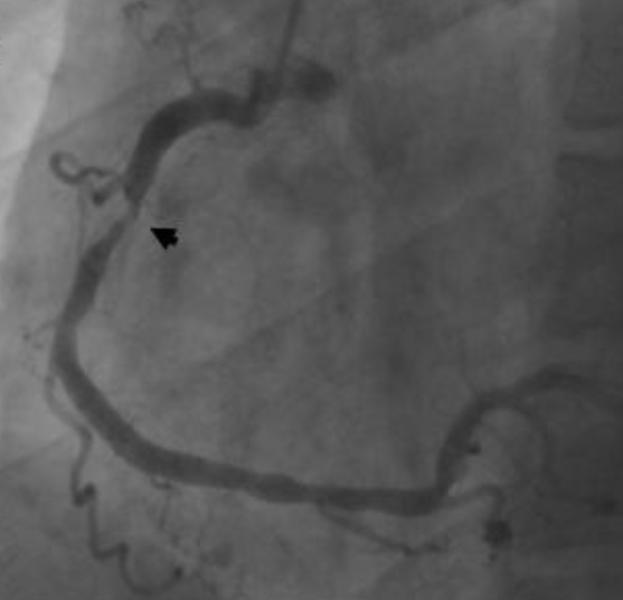
Coronary angiography is unable to accurately predict the severity of vessel narrowing, suggesting fractional flow reserve (FFR) functional tests should be added to help determine if a patient needs revascularization. This was according to research presented from the IRIS FFR-DEFER trial at the American College of Cardiology's (ACC) 61st Annual Scientific Session this week in Chicago.

The cost to place an implantable cardioverter-defibrillator (ICD) increased by $844 per case after a new requirement from the Centers for Medicare and Medicaid Services (CMS) went into effect in February 2010, which has added little additional benefit for patients. This was according to research presented during the American College of Cardiology’s 61st Annual Scientific Session in Chicago this week.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...

March 27, 2012 – Researchers found the antiplatelet drug abciximab significantly decreased damage to the heart muscle in patients with ST-segment-elevation myocardial infarction (STEMI), the most severe type of heart attack. Results of INFUSE-AMI trial were presented at the American College of Cardiology’s 61st Annual Scientific Session and published simultaneously in the March 25 online issue of the Journal of the American Medical Association. Researchers also found that clot aspiration did not significantly reduce the damage to the heart muscle.
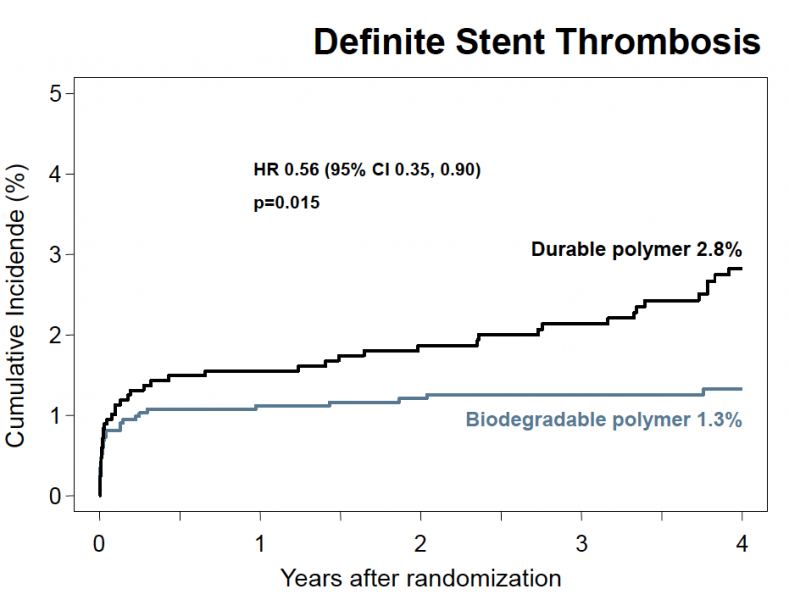
Biodegradable polymer drug-eluting stents (DES) provide better long-term safety and efficacy than durable polymer DES, according to findings from an analysis of three major clinical trials — ISAR-TEST 3, ISAR-TEST 4 and LEADERS. The data were presented at at the American College of Cardiology’s 61st Annual Scientific Session.
March 26, 2012 — ScImage Inc. launched a new cloud electrocardiogram (ECG) management service as an extension of its PicomCloud cloud picture archiving and communications system (PACS) at the American College of Cardiology's (ACC) 61st Annual Scientific Session in Chicago.


 March 29, 2012
March 29, 2012










