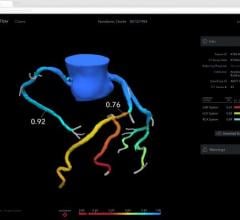
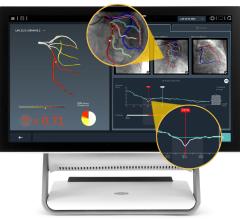
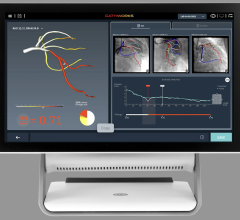
March 29, 2012 — St. Jude Medical Inc. announced it has received U.S. Food and Drug Administration (FDA) clearance for multiple enhancements to its PressureWire Fractional Flow Reserve (FFR) measurement guidewire. FFR measurement identifies the severity of narrowings in the coronary arteries and allows for a more effective assessment of coronary lesions, or blockages, resulting in more accurate diagnosis and improved appropriate treatment of coronary artery blockages.
The PressureWire with Agile Tip was designed to replicate the performance of standard percutaneous coronary intervention (PCI) guidewires and delivers exceptional handling even in the most challenging anatomies. Available in both of the company’s Aeris (wireless) and Certus PressureWire models, this eighth-generation guidewire also can deliver interventional tools more easily for faster and more cost-effective PCI.
Another new feature in the next-generation PressureWire is a proprietary hydrophilic coating that reduces friction within guide catheters and stent delivery catheters, allowing for easier deployment of stents and coronary balloons.
“In the evolution of our PressureWire family, we have long sought to match or exceed the handling performance of conventional workhorse PCI guidewires, a significant challenge owing to the presence of the pressure sensor and signal cables,” said Frank Callaghan, president of the St. Jude Medical Cardiovascular Division. “Based on the clinical feedback obtained to date, this eighth generation family of guidewires gives us confidence we have risen to the challenge.”
When integrated into routine lab procedures, measuring FFR, as shown by the FAME (Fractional Flow Reserve versus Angiography for Multivessel Evaluation) study sponsored by St. Jude Medical, reduces the risk of death or heart attack by 34 percent. PressureWire technology from St. Jude Medical was used exclusively in the FAME and FAME II trials and has been proven in more than 500 additional studies. Using PressureWire Aeris or PressureWire Certus to measure FFR helps ensure access to accurate information in the cath lab, increasing the efficiency and efficacy of coronary revascularization.
For more information: www.sjm.com


 February 03, 2026
February 03, 2026