November 2, 2012 – A clinical trial that compared catheter-based PFO closure using an investigational device found that there was no significant reduction in ischemic and bleeding events compared to standard medical therapy; stroke risk was non-significantly reduced with device therapy.

November 2, 2012 — Patients who receive a drug-eluting stent (DES) and demonstrate low levels of platelet inhibition are more likely to have blood clots form on the stent and suffer a possible heart attack; conversely, patients with higher levels of platelet inhibition are at greater risk for bleeding complications.
Several features/plug-ins have been added to aycan’s OsiriX PRO workstation to significantly expand its functionality. The new features will be showcased at Radiological Society of North America (RSNA) 2012, including the new aycan mobile iPad app, advanced hanging protocols, vessel analysis, 4-D ROI statistics and ejection fraction.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
November 1, 2012 — TomTec Imaging Systems GmbH announced U.S. Food and Drug Administration (FDA) 510(k) clearance for its new 2-D Cardiac Performance Analysis MR (2-D CPA MR) software solution for magnetic resonance imaging (MRI).
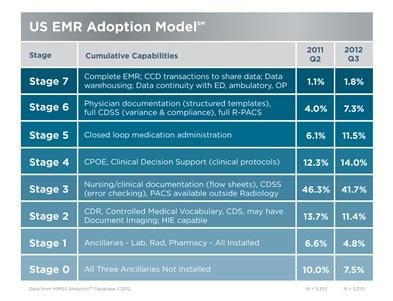
November 1, 2012 — HIMSS opposes the Oct. 4 call from four House Republican leaders for the Department of Health and Human Services (HHS) to “immediately suspend the distribution of incentive payments until [the department] promulgates universal interoperable standards.”
Medtronic Inc. announced promising data from the Engager European Pivotal Trial for the investigational Medtronic Engager transcatheter aortic valve implantation (TAVI) system. The first results from the multi-center trial support the safety and clinical performance of the valve, which uses a transapical delivery approach to treat patients with severe aortic stenosis who were at high or extreme risk for surgical aortic valve replacement (SAVR).
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
November 1, 2012 — It looks as though drug-eluting balloons (DEBs), already widely used in Europe, are set to burst into the global market as heart conditions become a more common problem, states a new report by healthcare experts GBI Research.
November 1, 2012 — Terumo Interventional Systems announced the nationwide availability of its new Pinnacle Precision Access System, a new vascular access system specifically designed for smooth, efficient and reliable peripheral vascular access.

November 1, 2012 — InspireMD Inc. announced that its proprietary MGuard embolic protection stent (EPS) was shown to be significantly superior when compared to standard bare metal and drug-eluting stents in achieving complete ST resolution and restoring normal blood flow in a major study of 432 randomized patients undergoing emergency coronary intervention for potentially fatal heart attacks.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...

October 31, 2012 — Boston Scientific Corp. received CE mark approval for the Synergy everolimus-eluting platinum chromium (PtCr) coronary stent system featuring an ultra-thin abluminal (outer) bioabsorbable polymer coating.

Breast cancer patients who receive radiation treatment do not have a higher risk of long-term cardiac morbidity when compared to patients undergoing modified radical mastectomy (MRM), according to research presented at the American Society for Radiation Oncology’s (ASTRO’s) 54th Annual Meeting. This is the first study to document comprehensive, late cardiac outcomes 25 years after breast cancer treatment.
Maquet Cardiovascular received 510(k) clearance from the U.S. Food and Drug Administration (FDA) and CE mark approval for its new Sensation Plus 7.5 French 40 cc intra-aortic balloon (IAB) catheter. This new larger-volume, fiber-optic IAB catheter will allow clinicians to provide a higher-efficacy IAB counterpulsation therapy “at the speed of light” to smaller patients.
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
W. L. Gore & Associates (Gore) responded to initial results reported in St. Jude Medical Inc.’s RESPECT clinical trial. The RESPECT study investigated whether transcatheter closure of patent foramen ovale (PFO) using St. Jude’s Amplatzer PFO Occluder device is safe and effective compared to best medical therapy in the prevention of recurrent cryptogenic stroke. Gore is concurrently conducting its Gore REDUCE Clinical Study using both the Gore Helex Septal Occluder and, as reported earlier this week, the new Gore Septal Occluder, in patients with PFO and a history of cryptogenic stroke or imaging-confirmed transient ischemic attack (TIA).

St. Jude Medical Inc., a global medical device company, announced that fractional flow reserve (FFR)-guided treatment using PressureWire was cost effective for coronary interventions when compared to the best available medical therapy. Cost utility analysis data from the FAME II trial was presented as a late-breaking clinical trial at the 24th annual Transcatheter Cardiovascular Therapeutics (TCT) scientific symposium, sponsored by the Cardiovascular Research Foundation.

Elixir Medical Corp. announced enrollment completion of the 120-patient, pivotal clinical trial evaluating the safety and efficacy of the DESolve Novolimus-Eluting Bioresorbable Coronary Scaffold System. The scaffold is designed to resorb in the body within one to two years after implantation and return the patients’ coronary vessel to de novo state.

 November 02, 2012
November 02, 2012