The Philips IntelliSpace Portal is an advanced visualization solution designed to simplify the way radiologists work, think and care for patients by delivering on the promise of real-time radiology.
October 29, 2012 — At RSNA 2012, GE Healthcare will unveil innovations in healthcare IT, including enhancements to Centricity PACS (picture archiving and communication system) and PACS-IW. The PACS-IW system aligns advanced visualization, breast imaging and intelligent tools with a unified desktop.
Boston Scientific Corporation has received regulatory approval to market the Reliance 4-Front lead, its next generation implantable defibrillation lead now available in Europe and Asia. Defibrillation leads are insulated wires that connect an implantable cardioverter defibrillator (ICD) or cardiac resynchronization therapy defibrillator to the heart for treatment of heart failure and sudden cardiac arrest.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
October 29, 2012 — Brainlab released Buzz Digital OR, a major step forward in information integration for the surgical suite. Buzz encompasses a vast spectrum, from pure DICOM viewing to complete digital operating room (OR) functionality, including video management and documentation.
Covidien announced final 12-month results from its DEFINITIVE LE (Determination of Effectiveness of SilverHawk/TurboHawk Peripheral Plaque Excision Systems for the Treatment of Infrainguinal Vessels/Lower Extremities) study.
Biotronik began its European market release of BioMonitor, an implantable cardiac device designed for accurate and reliable monitoring and management of patients with atrial fibrillation (AF) or unexplained syncope.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
October 25, 2012 — Lantheus Medical Imaging Inc. announced it has extended its contract with Nordion to supply molybdenum-99 (Mo-99) for use in its TechneLite (Technetium Tc-99m) generators.
October 25, 2012 — InterSystems Corp., a provider of software for healthcare interoperability, and eHealth Technologies, a provider of imaging solutions for electronic health records (EHR) and health information exchange (HIE) solutions, entered a partnership to provide single-click access to diagnostic-quality images via the InterSystems HealthShare strategic informatics platform.
Avinger Inc., a medical device manufacturer of multi-functional catheters for crossing CTOs in patients with peripheral artery disease (PAD), announced its CONNECT II global clinical trial results at the Vascular Interventional Advances (VIVA) Conference 2012 in Las Vegas, Nev.
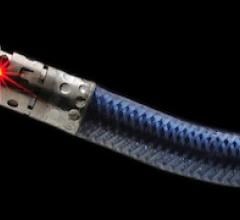
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
An online poll of physicians who lead hospitals, health systems and large group practices across the United States conducted after the first presidential debate found more preferred Republican challenger Mitt Romney's plan to reform Medicare than President Barack Obama's, with many still undecided.
October 23, 2012 — A study found that a tailored therapy approach that adjusts the loading dose of clopidogrel according to measured platelet reactivity is superior to prasugrel therapy in reducing high platelet reactivity (a risk factor for worse outcomes) in patients with acute coronary syndrome (ACS). Results of the trial were presented at the 24th annual Transcatheter Cardiovascular Therapeutics (TCT) scientific symposium.
October 23, 2012 — In the DEBATE-SFA trial, researchers found that the use of a paclitaxel drug-eluting balloon (DEB) to widen the artery prior to implanting a self-expanding nitinol stent yielded a lower restenosis rate after one year. Results of the trial were presented at the 24th annual Transcatheter Cardiovascular Therapeutics (TCT) scientific symposium.
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
October 22, 2012 — W. L. Gore & Associates announced that the U.S. Food and Drug Administration (FDA) has approved the use of the new Gore septal occluder for inclusion in the Gore REDUCE clinical study.
At RSNA 2012, GE Healthcare will be showcasing a comprehensive portfolio of ultrasound products for use across a broad range of patient exam types. Among the products to be highlighted will be the newly enhanced Logiq E9, Logiq S8 and others in the LOGIQ family.
NEC Display Solutions of America announced the U.S. Food and Drug Administration (FDA) 510(k) market clearance of the 21.3-in MultiSync MD211G3 medical diagnostic, flat panel display for the displaying and viewing of digital mammography images for diagnosis by trained physicians.

 October 29, 2012
October 29, 2012