Abbott has received Conformite Europeene (CE) marking for the Architect Galectin-3 assay, a blood test to aid doctors in assessing the prognosis of patients diagnosed with chronic heart failure.
The Obama administration has released the long-anticipated budget proposal for fiscal 2014. Although the document did not include specific reductions in reimbursement for advanced diagnostic imaging services, the fiscal 2014 budget would largely exclude certain imaging services from the in-office ancillary services exception (IOASE) to the Stark self-referral law and calls for prior authorization for advanced imaging services.
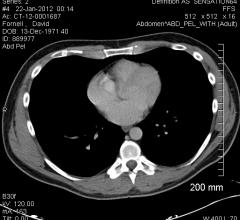
One venous puncture, rather than two, is a safe and effective approach to intravascular ultrasound-guided inferior vena cava filter placement in critically-ill patients according to a new study shows. Inferior vena cava filter placement is done to prevent or treat pulmonary emboli or deep venous thrombosis.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Multi-detector computed tomography (MDCT) is a better way to measure annular size in patients with aortic stenosis who are candidates for transcatheter aortic valve implantation (TAVI) than two dimensional echocardiography according to a new study.

The staffing firm SpringBoard Healthcare is conducting a wage information survey to gather information about cath lab’s across the country. Reader input is needed to create a clear picture of trends in the industry.
Kidney failure patients on dialysis derive long-term benefit from the minimally invasive placement of a stent that improves the function of dialysis access grafts, according to 12-month clinical trial results being presented at the Society of Interventional Radiology's 38th Annual Scientific Meeting in New Orleans.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Accumetrics Inc. announced an expansion in the intended use for the CE marked VerifyNow P2Y12 Test. Physicians can now use the test results to assess whether a patient may be at greater risk for both bleeding or ischemic events, as an aid to manage therapeutic treatment decisions and accurately assess the antiplatelet effect from P2Y12 inhibitors (such as clopidogrel, prasugrel and ticagrelor).
Maquet Cardiovascular LLC has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) and European CE mark approval for its new Air-Band Radial Compression Device. Indicated to assist hemostasis of the radial artery after a transradial procedure, the device is designed to compress the radial artery puncture site while maintaining site visibility and a secure fit around the wrist. Air-Band will be commercially available in the European Union later this month and in the United States in April.
Improving patient safety while offering clinicians a more complete picture during interventional procedures, Toshiba America Medical Systems Inc. will showcase Spot Fluoroscopy for its Infinix-i vascular X-ray systems at this year’s Society of Interventional Radiology (SIR) 2013 annual meeting.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Tyrx announced the first implantation of its new AigisRx R Fully Resorbable Antibacterial Envelope has taken place at the Quebec Heart and Lung Institute in Quebec City, Canada by Dr. François Philippon. Tyrx announced on Jan. 31, 2013 that it had received a license from Health Canada to market its AigisRx R Fully Resorbable technology.
Cook Medical has launched the VIVO clinical research study to evaluate the safety and effectiveness of the Zilver Vena Venous Self-Expanding Stent in the treatment of symptomatic iliofemoral venous outflow obstruction. This disease is characterized by leg pain, throbbing, swelling and skin discoloration in the legs.
Nanomix Inc. has initiated clinical testing in the United States to evaluate the omega-3 cardiac panel. The panel quantitatively tests whole blood specimens for levels of cardiac troponin I (cTnI), myoglobin and H-FABP, biomarkers often used as an aid in the diagnosis of myocardial infarction (MI). The initial testing will be conducted with approximately 170 normally healthy subjects and will provide baseline performance information that will form the basis of future registration clinical studies.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
At Medanta Hospital in Gurgaon, India, five EchoBox devices were connected to five GE Vivid ultrasound systems, streaming live echo images over the web to experienced sonographers located in the United States.
The Massachusetts General Hospital officially announced the launch of the Mass General Institute for Heart, Vascular and Stroke Care, one of the only institutes in the world to integrate cardiovascular and cerebrovascular care.
The U.S. Food and Drug Administration (FDA) issued a second complete response letter regarding a supplemental new drug application (sNDA) for Xarelto (rivaroxaban) for the reduction of the risk of cardiovascular events in patients with acute coronary syndrome (ACS). Janssen Research and Development LLC is evaluating the letter and will respond to the agency's questions.


 April 18, 2013
April 18, 2013