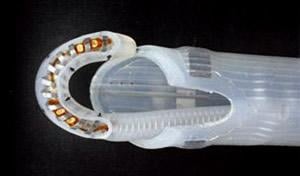
nContact Inc. said it received conditional approval for an investigational device exemption (IDE) from the U.S. Food and Drug Administration (FDA) to begin enrollment in the CONVERGE trial, a multicenter, prospective, randomized study evaluating patients with symptomatic persistent atrial fibrillation (AF). AF is the most common cardiac arrhythmia, a condition that disrupts the ability of the atria (upper chambers of the heart) to beat regularly and pump blood efficiently. The CONVERGE study, designed to investigate the epicardial/endocardial Convergent Procedure, combines the cardiac ablation expertise, techniques, and technologies of both electrophysiologists and cardiothoracic surgeons.
Biosense Webster Inc. announced the 12-month safety and effectiveness results of the Thermocool Smarttouch catheter and software module in the treatment of symptomatic, drug refractory, paroxysmal atrial fibrillation (AF) from the SMART-AF investigational device exemption (IDE) clinical trial. The results were presented at the Heart Rhythm Society’s 34th Annual Scientific Sessions by Andrea Natale, M.D., a member of the study advisory committee and executive medical director of the Texas Cardiac Arrhythmia Institute at St. David’s Medical Center in Austin, Texas.
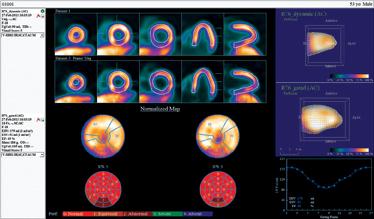
The next generation of positron emission tomography (PET) imaging agents will herald an age when PET will eclipse single photon emission computed tomography (SPECT) as the “go to” modality for molecular imaging. It will do so by enabling personalized medicine through precision diagnostics, the ability to be delivered cost-effectively in a manner with less radiation to patients, by leveraging hardware advances already being commercialized, and by taking advantage of the extra throughput capacity present in the U.S. installed base of PET/CT scanners.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Toshiba America Medical Systems Inc. offers electrophysiology (EP) clinicians an Infinix-i cardiovascular X-ray system tailored for EP procedures with a new package of features, accessories and technologies. This package maximizes room utilization, improves workflow and enhances safety.

The first patient tests of a new leadless pacemaker show that the device can deliver the same life-saving therapy as a traditional single-chamber pacemaker without potential lead-related complications. The findings are part of the LEADLESS trial and were released at Heart Rhythm 2013, the Heart Rhythm Society’s 34th Annual Scientific Sessions.
Wearable cardioverter defibrillators (WCD) can be an effective therapy option for patients with a transient or undefined arrhythmic risk, according to the WEARIT-II Registry, the largest prospective study to track patients with the device in a real-world setting. The new findings show that WCDs can serve as a bridging therapy and help avoid unnecessary permanent implantation of defibrillators in patients who ultimately may not need them.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Biotronik announced the U.S. Food and Drug Administration (FDA) granted approval for its Ilesto 7 implantable cardioverter-defibrillator/cardiac resynchronization therapy defibrillator (ICD/CRT-D) series. The devices are smaller, thinner and lighter than previous systems.
The American Society of Nuclear Cardiology (ASNC) testified at a U.S. House Ways and Means Health Subcommittee hearing that examined options for repealing the sustainable growth rate (SGR) formula and reforming the Medicare physician payment system to reward quality and value in patient care. Kim Allan Williams, M.D., ASNC past-president and a current member of the society’s health policy steering committee, testified as a witness in the hearing on May 7, 2013. Williams currently serves as the Dorothy Susan Timmis endowed professor and chairman of the division of cardiology at Wayne State University School of Medicine.

During the American College of Cardiology 2013 (ACC.13) annual meeting in March, vendors discussed several trends they are observing in the cardiac ultrasound market and displayed the latest echo advances.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...

The development of 3-D transesophageal echo (TEE) just a few years ago has enabled a new generation of interventional procedures to be performed, which otherwise would have been extremely difficult or impossible. With live 3-D TEE, physicians can see cardiac structures and function, as well as real-time displays of the beating heart, from new perspectives.

The Aspirus Heart & Vascular Institute (AH&VI) services 14 counties in north central Wisconsin and an additional seven counties in the Upper Peninsula of Michigan. The institute receives patients from five hospitals within its system and approximately 10 other hospitals from within this service area. With such a large, mostly rural population — patients come from as far away as 80 miles or more — the organization needed to find a way to more efficiently serve its ST-elevation myocardial infarction (STEMI) and heart valve repair patients.
When cath labs begin performing radial access procedures, both the staff and the operators need to keep an open mind and recognize that it takes time for everyone in the lab to become proficient in doing things in a different way. Learning a new skill can take time and dedicated effort, and as long as one recognizes that, a fledgling transradial program can flourish rather than be abandoned. We offer the following tips for consideration based on our own experience.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...

There is a growing trend in the use of small, portable extracorporeal membrane oxygenation (ECMO) systems for hemodynamic support. In years past, ECMO systems were a tool of the operating room, primarily for bypass surgery. Older systems required a perfusionist to watch the machine 24 hours a day while it was in operation. Today’s ECMOs use consoles that are much easier to use and do not require a full-time person to monitor. Also, as the technology has been miniaturized, simplified and now uses a percutaneous access system, ECMOs are being used outside the OR, including the cath lab, when a large amount of support is needed that cannot be provided by IABPs or pVADs.

Since the 1970s, intra-aortic balloon pumps (IABPs) have been the gold standard of minimally invasive hemodynamic support, but more recently developed percutaneous ventricular assist devices (pVAD) are offering an alternative. Clinical data shows pVADs offer more support than IABPs, but their much higher price tag currently restricts their use to niche applications, usually after an IABP fails to deliver enough support.
Live demonstration, transmission or recording of transcatheter aortic valve replacement (TAVR) has been found feasible and safe, even for the typically high-risk patients who undergo the procedure. Results of the VERITAS late-breaking clinical trial, presented at the SCAI 2013 Scientific Sessions, confirmed via comparison of on- and off-camera case studies that recording procedures did not pose any additional risk or complications to patients.

 May 14, 2013
May 14, 2013









