Aptus Endosystems Inc. announced that it received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its 28 mm Tip Reach Heli-FX Guide. A line extension of the original Heli-FX System, the new product enhances treatment of wide neck abdominal aortic aneurysms (AAA). The announcement comes ahead of this week’s Society of Vascular Surgery (SVS) Vascular Annual Meeting in San Francisco where the company will be launching this device as well as the recently approved Heli-FX Thoracic System.
Final six and 12-month results of the DIRECT first-in-man clinical study were presented by study principal investigator Dr. Mark Webster at the late-breaking clinical trials session of the EuroPCR meeting in Paris, France. No patients experienced clinically-driven TLR, TVR or MACE at six months, with results sustained through 12 months. It is believed the Svelte drug-eluting stent is the first ever to achieve 0 percent clinically-driven MACE through 12-months in a independent core-lab and DSMB adjudicated clinical study.
The Idaho Health Data Exchange (IHDE) and St. Luke’s Health System (SLHS) have launched Image Exchange viewing capability by eHealthTechnologies.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Cardiovascular Systems Inc. (CSI) announced that the first patient has been enrolled in its post-market clinical study, LIBERTY 360°. The study is evaluating the acute and long-term clinical and economic outcomes of CSI’s orbital atherectomy system in treating peripheral arterial disease (PAD). Additionally, LIBERTY 360° is the first study of its kind to compare orbital atherectomy to all other PAD treatment options in a difficult-to-treat patient population. Cezar Staniloae, NYU Medical Center / New York Cardiovascular Associates, N.Y., the study’s principal investigator, performed the first procedure.
Biosensors International Group Ltd. has entered into a licensing agreement with Eurocor GmbH, a group company of Opto Circuits (India) Ltd. for their drug eluting balloon (DEB) technology and related intellectual property (IP) rights in relation to the treatment of both coronary and peripheral artery disease. As a first step in this process, an original equipment manufacturer (OEM) arrangement is being implemented, whereby Biosensors will market and sell, under its own brand, DEBs manufactured by Eurocor.
June 19, 2013 — Coronary artery disease (CAD) is one of the world’s most prevalent and silent killers. Positron emission tomography (PET), which images miniscule abnormalities in cellular metabolism, can tip off clinicians about cardiac disasters waiting to happen — including sudden death from a heart attack — better than standard angiography, researchers revealed at the Society of Nuclear Medicine and Molecular Imaging’s 2013 Annual Meeting.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Magnetic resonance imaging (MRI) scans of children who have had chemotherapy can detect early changes in their hearts finds research in biomed Central's open access journal Journal of Cardiovascular Magnetic Resonance.
Valtech Cardio Ltd. announced that two patients diagnosed with severe mitral regurgitation (MR) have been treated successfully with its Transfemoral Cardioband Annuloplasty System.
Criteria for evaluating operator competency for performing coronary interventions should be expanded beyond procedure volume and include an evaluation of risk-adjusted outcomes, periodic case reviews of patient selection and other factors, according to a new report from the American College of Cardiology, the American Heart Association and the Society for Cardiovascular Angiography and Interventions.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...

Preliminary results from the ADVISE (Adenosine Vasodilator Independent Stenosis Evaluation) II trial confirm prior retrospective publications and demonstrate the clinical usefulness of an iFR/fractional flow reserve (FFR) hybrid approach to simplify lesion assessment and to save the use of hyperemic drugs in a significant number of patients. It was also announced that this hybrid iFR/FFR strategy, along with intravascular ultrasound (IVUS) guidance, will be used in the multi-center SYNTAX2 trial in Europe starting later this year.
Agfa began its global release of ICISTM View 3.0, the medical images and results viewer for the comprehensive ICIS enterprise imaging solution. The ICIS patient imaging data platform is a fundamental part of the company’s global e-health strategy. Building on its leadership in enterprise imaging management and web-enabled technology, Agfa HealthCare’s ICIS View 3.0 allows clinicians, specialists, and all other stakeholders to access all patient imaging data from any PACS or VNA, using a single viewer, to support continuity and productivity of patient care. This cost- effective solution leverages the hospital’s existing investment in technology, and provides access to the most current and relevant patient imaging data directly from the source.
St. Jude Medical Inc. announced U.S. Food and Drug Administration (FDA) approval to begin the EnligHTN IV Renal Denervation Study, the first U.S. trial using the EnligHTN Multi-Electrode Renal Denervation System to treat patients with drug-resistant high blood pressure.
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
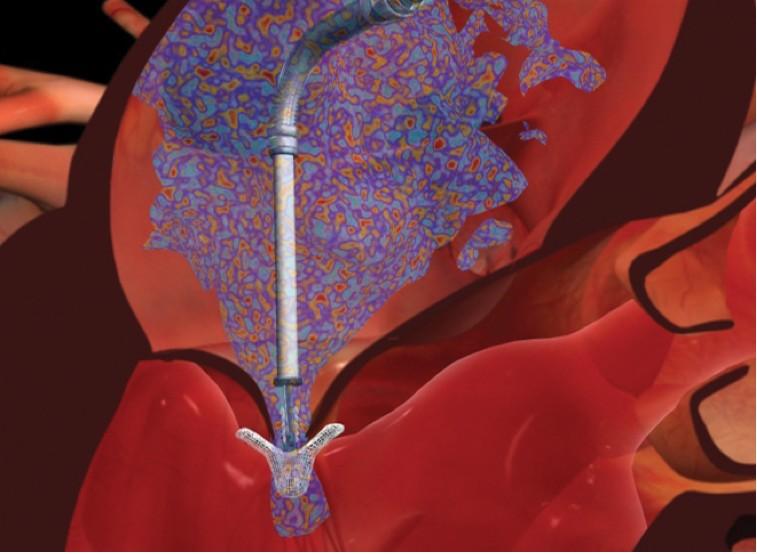
Abbott has announced publication of positive outcomes from two European post-approval studies of the catheter-based MitraClip therapy for the treatment of mitral regurgitation (MR). Results from ACCESS-EU, a European prospective study that enrolled 567 patients at 14 sites, have been published in the Journal of the American College of Cardiology. In addition, findings of the investigator-sponsored German TRAnscatheter Mitral Valve Interventions (TRAMI) registry, which enrolled 1,064 patients at 20 German sites, were recently published in EuroIntervention.
Tryton Medical Inc. announced that the Nordic-Baltic Bifurcation Study Group will investigate the Tryton Side Branch Stent. The trial is a prospective, controlled, randomized, multicenter clinical study examining the role of final kissing balloon inflations in patient outcomes. A total of 150 patients will receive the Tryton Side Branch Stent with a drug-eluting stent, with evaluation by intravascular optical coherence tomography (OCT) imaging technology.
Mirada Medical collaborators has presented new positron emission tomography/magnetic resonance imaging (PET/MRI) research. The software supports both the hardware hybrid scanners and provides validated deformable registration for software based PET/CT (computed tomorgraphy)/MRI.

 June 24, 2013
June 24, 2013








