
The tiny device, about the size of the head of a pen, can be delivered via a delivery catheter through the venous system and placed directly into the myocardium of the right ventricle.
Neovasc Inc. announced that the first patent covering the company's innovative Tiara transcatheter mitral valve replacement technology has been issued by the U.S. Patent and Trademark Office. The new patent protects key aspects of the Tiara mitral valve prosthesis.
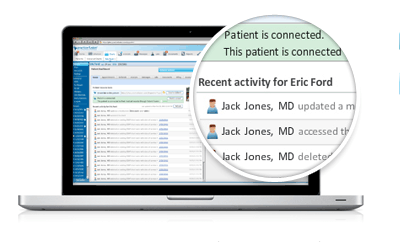
As health IT leaders make their way to New York for the New York eHealth Collaborative (NYeC) Digital Health Conference, Practice Fusion, the nation's largest healthcare platform, is looking at the key healthcare trend from 2013 and predicts what they might mean looking forward.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Professional cardiovascular societies and many working cardiologists question the U.S. Food and Drug Administration’s (FDA’s) recent recommendation that patients undergo genetic testing before taking Plavix (clopidogrel bisulfate).
AngioScore, Inc., a developer of novel angioplasty catheters for use in the treatment of cardiovascular disease, announced detailed preliminary clinical trial results for the world’s first drug-coated scoring balloon catheter.
Clear Image Devices LLC (CiD) announced the release of its step platform to be used for standing patient ultrasound venous insufficiency studies. The Ultrasound Exam Steps enable sonographers to place the patient in the optimal position for safe, easy capture of the highest quality venous images of the upper and lower leg.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
To help reduce the burden of cardiovascular disease, the nation's leading killer, New York-Presbyterian Hospital and Weill Cornell Medical College have created the Dalio Institute of Cardiovascular Imaging. Raymond T. Dalio, a life trustee of New York-Presbyterian Hospital, has made a gift of $20 million through his Dalio Foundation in support of the institute.
The Eurozone economic slowdown has made it difficult for hospitals, particularly in Southern Europe, to procure new imaging modalities. With streamlined budgets and escalating number of medical procedures, the need to spend less on technologies while gaining the maximum benefit from them has sustained demand for high-quality refurbished imaging equipment.

The Society for Cardiovascular Angiography and Interventions (SCAI) published a first-of-its-kind paper defining best practices for the use of the transradial approach for diagnosing and treating blocked heart arteries.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
InspireMD Inc., a developer of embolic protection stents, announced 12-month results from the MGuard for Acute ST Elevation Reperfusion (MASTER) trial demonstrating that the MGuard outperformed bare metal and drug-eluting stents in all-cause mortality in ST segment elevation myocardial infarction (STEMI) patients.
Lantheus Medical Imaging, Inc., a developer, manufacturer and distributor of diagnostic imaging agents, announced preliminary results from the first of two planned Phase 3 trials to assess the diagnostic efficacy of flurpiridaz F 18, an imaging agent used in positron emission tomography (PET) myocardial perfusion imaging (MPI) for the detection of coronary artery disease (CAD).
Petr Neuzil, M.D., Nemocnice Na Homolce Hospital, Prague, Czech Republic, presented three-month results from the WAVE II study and complete six-month results for the WAVE I (first-in-man) at the Transcatheter Cardiovascular Therapeutics conference (TCT 2013).
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...

No law prevents doctors from freely prescribing U.S. Food and Drug Administration (FDA)-approved drugs and devices for off-label uses, yet regulators continue to aggressively pursue civil and criminal enforcement of perceived violations, warns Patrick J. Hurd, senior counsel with LeClairRyan in Washington, D.C. and Norfolk, Va. in the November 2013 edition of Westlaw Journal Pharmaceutical.
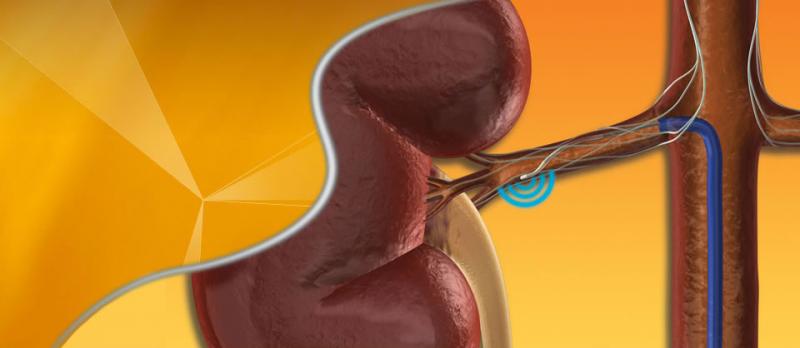
Medtronic Inc. announced that the U.S. Food and Drug Administration (FDA) has approved an Investigational Device Exemption (IDE), allowing the company to initiate SYMPLICITY HTN-4, the first randomized trial to investigate renal denervation for the treatment of moderate uncontrolled hypertension in U.S. patients.
Aycan will feature its mobile iPad application, which is designed to quickly, easily and securely transfer DICOM images from hospitals and imaging centers to on-call and other radiologists and referring physicians with an iPad, at the Radiological Society of North America Annual Meeting (RSNA 2013) in Chicago.

 November 15, 2013
November 15, 2013










