New study results validate the effectiveness of the Saranas Early Bird Bleed Monitoring System to sense bleeding events during endovascular-related procedures by using sensors to detect relative changes in tissue bioimpedance. The study enrolled 60 patients from five sites who underwent an endovascular procedure and detected bleeding in more than half of patients. The results of this study were presented as late- breaking clinical research on at the Society for Cardiovascular Angiography Interventions (SCAI) 2019 Scientific Sessions, May 19-22 in Las Vegas.
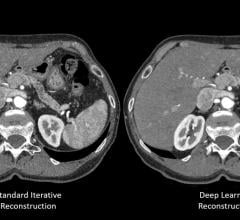
Canon Medical Systems USA Inc. has received 510(k) clearance on its new deep convolutional neural network (DCNN) image reconstruction technology, dubbed Advanced Intelligence Clear-IQ Engine (AiCE). AiCE uses a deep learning algorithm to differentiate signal from noise so that it can suppress noise while enhancing signal.
Results from a first-in-man proof of concept study found occlusion of the superior vena cava (SVC) rapidly and effectively reduces pressure and volume in the heart. This is the first study targeting the SVC as a therapeutic area for heart failure patients to reduce cardiac filling pressures without reducing cardiac output or systemic blood pressure. Data was presented as late-breaking clinical research at the Society for Cardiovascular Angiography and Interventions (SCAI) 2019 Scientific Sessions, May 19-22 in Las Vegas.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Initial results from the AVIATOR 2 international registry were presented as late-breaking clinical science at the Society for Cardiovascular Angiography and Interventions (SCAI) 2019 Scientific Sessions, May 19-22 in Las Vegas. The AVIATOR 2 is a multicenter prospective observational study of patients with atrial fibrillation (AF) undergoing percutaneous coronary intervention (PCI) in 11 international sites. The use of a novel smartphone-based survey shows potential to improve outcomes by accessing the patient need for new tools to quantify risk.
A prospective, multicenter, multinational study examines how fractional flow reserve (FFR) can impact treatment plans and outcomes in patients with stable coronary artery disease (CAD) or acute coronary syndrome (ACS). Results showed more than one-third of the patients’ initial treatment plans changed after FFR. The findings from the global registry were presented as late-breaking clinical research at the Society for Cardiovascular Angiography and Interventions (SCAI) 2019 Scientific Sessions, May 19-22 in Las Vegas.
Akshay Khandelwal, M.D., director of medical operations at the Henry Ford Heart and Vascular Institute, Detroit, and ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Quantum Genomics announced the enrollment of the first patient in its QUORUM Phase IIb study of its lead clinical candidate, firibastat, in patients with heart failure after acute myocardial infarction (AMI). Firibastat is a first-in-class brain aminopeptidase A inhibitor, being developed for the treatment of resistant hypertension and heart failure.
June 14, 2019 – A late-breaking study examined the effects of intravascular ultrasound (IVUS) guided drug-eluting stent ...
June 14, 2019 – An expert consensus statement provides recommendations for optimizing the financial operations of the ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
Bardy Diagnostics Inc. announced that its Carnation Ambulatory Monitor (CAM) was recognized with the “Best New Diagnostic Technology” award in the 2019 MedTech Breakthrough Awards Program. MedTech Breakthrough is an independent organization that recognizes the top companies and solutions in the global health and medical technology market. BardyDx earned the distinction for its P-wave centric ambulatory cardiac patch monitoring and arrhythmia detection technology.
Silk Road Medical Inc. announced the presentation of real-world data for the treatment of patients with carotid artery disease at risk for stroke at the Society for Vascular Surgery 2019 Vascular Annual Meeting (VAM), June 12-15 in National Harbor, Md. In a headline presentation, Mahmoud Malas, M.D., of the University of California, San Diego School of Medicine shared updated results for the ongoing TransCarotid Artery Revascularization (TCAR) Surveillance Project.
Three-dimensional (3-D) printing software and solutions company Materialise has received U.S. Food and Drug Administration (FDA) clearance for its Mimics Enlight cardiovascular planning software suite. The first release will support clinicians planning complex transcatheter mitral valve replacement (TMVR) procedures.
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
Orchestra BioMed Inc. announced it has formed a global strategic partnership with Terumo Corp. for development and commercialization of the Virtue Sirolimus-Eluting Balloon (SEB), one of Orchestra’s lead assets, in the percutaneous coronary and peripheral interventions field.

June 12, 2019 — Here is the list of the most popular content on the Diagnostic and Interventional Cardiology (DAIC) ...
Boston Scientific Corp. has initiated the OPTION trial to compare safety and effectiveness of the next-generation Watchman FLX left atrial appendage closure (LAAC) platform to first-line oral anticoagulants (OAC) for stroke risk reduction in patients with non-valvular atrial fibrillation (AF) who undergo a cardiac ablation procedure. OACs used in the trial will include direct oral anticoagulants (DOAC) and warfarin.


 June 18, 2019
June 18, 2019