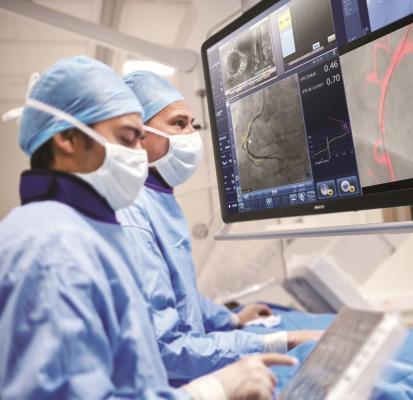
June 17, 2019 – A prospective, multicenter, multinational study examines how fractional flow reserve (FFR) can impact treatment plans and outcomes in patients with stable coronary artery disease (CAD) or acute coronary syndrome (ACS). Results showed more than one-third of the patients’ initial treatment plans changed after FFR. The findings from the global registry were presented as late-breaking clinical research at the Society for Cardiovascular Angiography and Interventions (SCAI) 2019 Scientific Sessions, May 19-22 in Las Vegas.
According to the Centers for Disease Control (CDC), CAD is the most common type of heart disease, killing more than 370,000 people a year in the United States. FFR is known to be an extremely accurate diagnostic tool for CAD and ACS patients. It offers a precise physiology technique to measure blood pressure and flow in a coronary artery, and has recently become a routine practice to help guide revascularization decisions for clinicians compared to angiography alone.
In this registry, for the first time, treatment plans for patients were recorded prospectively rather than retrospectively following angiography, prior to performing FFR. Treatment options included medical therapy, percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG). Standard pre-defined clinical outcomes were also identified and recorded.
The PRESSUREwire (Practical Evaluation of Fractional Flow ReservE (FFR) and its ASsociated Alternate IndiceS DUring Routine Clinical ProcEdures) study enrolled more than 2,200 subjects across 70 hospitals in 15 countries between October 2016 and February 2018. Treatment plans based on angiography alone changed in 34 percent of patients post-FFR. Results showed approximately 5 percent more patients were planned for medical management post-FFR than post-angiography, fewer patients were assigned to PCI treatment post-FFR and more patients were assigned to CABG. There were no serious adverse events related to FFR measurement.
“Our registry data further validates numerous previous studies and reinforces the importance of using coronary physiology guidance in conjunction with angiography to guide the patient treatment plan,” said lead author Erick Schampaert, M.D., Hospital Sacre-Coeur De Montreal in Montreal, Canada. “Having accurate measurements can make a difference between a patient having an unnecessary invasive procedure or not intervening quickly enough when a patient does need further attention. Equipping physicians for better patient management has the potential to positively impact patient outcomes.”
The authors of the study note the importance of incorporating FFR in daily clinical practice and recommend interventionalists to rely less on angiography alone, when non-invasive documentation of ischemia is not available.
For more information: www.scai.org


 January 05, 2026
January 05, 2026 









