July 18, 2024 — Elucid, a pioneering AI medical technology company providing physicians with imaging analysis software based on ground truth histology to support diagnosis and treatment of cardiovascular disease, has initiated site recruitment for its Plaque as a Risk Factor for Evaluation of Vascular Events (PRE-VUE) CT Registry Study. The lead investigators for the study are Dr. Carlo De Cecco (Emory University, Atlanta) and Dr. Márton Kolossváry (Gottsegen National Cardiovascular Center, Budapest, Hungary).
PRE-VUE is an international, multicenter, retrospective trial evaluating the prognostic accuracy of Elucid’s next generation, histology-defined plaque analysis, PlaqueIQ, and investigational plaque-based FFRCT software in predicting major adverse cardiac events (MACE) in patients who have received clinically indicated coronary CT angiography (CCTA). The study will include at least 30 centers and a pool of at least 10,000 patients ages 18 and over, all of whom underwent CCTA with continuous follow-up for a minimum 36 months.
“This registry will aim to provide world-wide physicians the most accurate information on coronary plaque to improve cardiovascular risk prediction and support the selection of patient-specific treatment,” said Dr. De Cecco. “The ultimate goal is to positively impact cardiovascular health globally with a reduction in cardiovascular events."
Dr. Kolossváry added, “The PRE-VUE registry will empower us to see the true additive value of CT-derived, histologically validated plaque volumes in predicting future adverse cardiovascular events.”
Cardiovascular disease is the most common cause of death and disability globally, largely driven by myocardial infarction and ischemic stroke caused by atherosclerosis (plaque build-up in the arteries).1 Although plaque disruption, such as rupture, is the most common cause of arterial thrombotic events,2 traditional diagnostic methods for characterizing plaque use subjective visual assessments, which vary according to scanning protocol or interpreter and can omit critical patient risk information such as identifying vulnerable plaque types.3,2
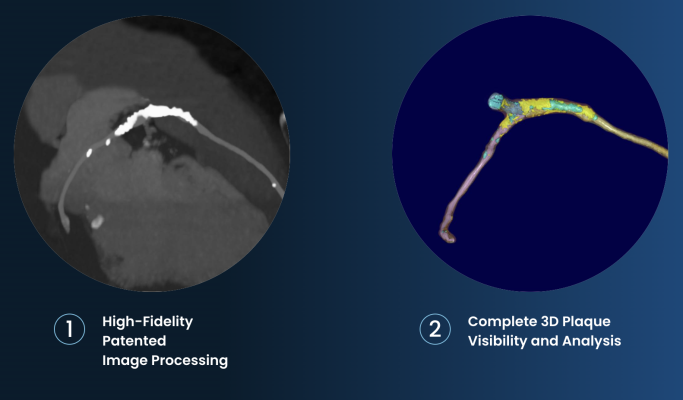
Elucid is addressing the need for new objective, non-invasive diagnostic methods that more accurately quantify and characterize plaque, including vulnerable plaques, based on actual histology (the microscopic structure of organic tissues excised from live patients). Elucid’s PlaqueIQ is the first and only histology-validated FDA-cleared software to non-invasively quantify and characterize non-calcified plaque and its components such as lipid-rich necrotic core (LRNC), giving potential insights into high-risk plaques, key drivers of risk of heart attack and stroke. The company is also pursuing an indication for non-invasive measurement of fractional flow reserve (FFRCT), uniquely derived from its plaque algorithm, to measure coronary blockages and the extent of ischemia.
“Characterizing anatomical and physiological parameters through the amount and type of plaque in a patient’s artery is essential to understanding their risk of heart attack and stroke. Elucid is committed to building a large repository of rigorous evidence to support our histology-based solutions, and the PRE-VUE trial is a testament to this,” said Scott Huennekens, executive chairman of the board, Elucid. “Building on the significant clinical study work we have done to date, this trial breaks new ground in our field, as it uniquely investigates a large and diverse patient population spread across a variety of international healthcare settings. We are grateful for the PRE-VUE investigators, who see great potential in our unique platform and non-invasive solutions that support clinical intervention decisions to halt and reverse trends in cardiovascular disease.”
For more information: www.euclid.com
Find more SCCT24 conference coverage here
References:
|
1 |
World Health Organization (WHO), Cardiovascular diseases (CVDs) fact sheet. 2017 23, April 2020; Available from: https://www.who.int/en/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds). |
|
2 |
A. Hafiane, Vulnerable plaque, characteristics, detection, and potential therapies, J. Cardiovasc. Dev. Dis. 6 (3) (2019). |
|
3 |


 February 02, 2026
February 02, 2026